Biden Administration secures supply of new COVID-19 therapeutic treatment
The Biden Administration announced that it has secured a supply of a recently authorized COVID-19 therapeutic treatment, announced the U.S. Department of Health and Human Services (HHS) in a news release.
HHS and the Department of Defense (DOD) collaborated to purchase 100,000 treatment courses of a second therapeutic treatment from Eli Lilly and Company. This treatment uses two monoclonal antibodies, bamlanivimab and etesevimab, to treat non-hospitalized, high-risk COVID-19 patients.
The U.S. Food and Drug Administration (FDA) issued emergency use authorization (EUA) for Lilly’s therapeutic of bamlanivimab and etesevimab on Feb. 9, 2021. The treatment is administered through an intravenous (IV) infusion and is intended for non-hospitalized patients with confirmed COVID-19 who are experiencing mild to moderate symptoms and are at high-risk for severe symptoms and hospitalization. The treatment uses a single dose for each patient.
The Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR), collaborated with the DOD Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) and the Army Contracting Command to provide $210 million for the initial purchase of up to 100,000 treatment courses of the bamlanivimab and etesevimab therapeutic. The agreement includes flexibility to purchase additional treatment courses as needed, up to a total of 1.2 million, through November 2021.
The recently authorized antibody therapeutic adds to the list of products available in the fight against the COVID-19 pandemic, including Lilly’s single monoclonal antibody therapy, bamlanivimab, and Regeneron Pharmaceuticals’ therapeutic that uses casirivimab and imdevimab. Both therapeutics received emergency use authorization in November 2020.
To help states and territories identify and allocate the treatment courses to non-hospital facilities that serve priority and underserved populations, HHS initiated a Special Projects for Equitable and Efficient Distribution (SPEED) program. Eligible facilities include nursing homes, assisted living facilities, federally qualified health centers, correctional facilities, dialysis centers, and other healthcare settings.
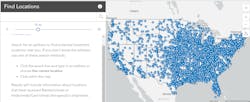
HHS also implemented a direct ordering system for healthcare facilities to order any of the available therapeutics, and all three remain free of charge to receiving sites. To help patients and healthcare providers find possible treatment locations for any of the available antibody therapeutic treatments, HHS created a treatment locator that provides information on where the medicines have been delivered.
Lilly developed the bamlanivimab and etesevimab treatment without federal support. The two monoclonal antibodies that make up the combination therapeutic were identified from blood samples taken from patients who recovered from COVID-19. Monoclonal antibodies, which mimic the human immune response, are produced outside of the body by a single clone of cells or a cell line with identical antibody molecules and then delivered to patients by infusion. The antibodies bind to certain proteins of a virus, reducing the ability of the virus to infect human cells.
In issuing EUAs for the monoclonal antibody therapeutics, FDA noted the potential for adverse events or side effects of bamlanivimab alone or when administered with etesevimab and in administering Regeneron’s casirivimab and imdevimab.