As a device that enters the body, a catheter has the potential to cause infection, while some designs and purposes present greater risk than others. Clinical staff training and education on proper hygiene practices, catheter component disinfection, safe catheter handling, and wearing of personal protective equipment (PPE) while caring for patients with indwelling catheters is of critical importance to minimizing the risk for contamination. Compliance monitoring and reporting is essential as well.
But at a time when there are too few nurses, not enough supplies, and a steady stream of new research around reducing catheter-associated infection risks, there is also a need for technology interventions that can augment the human element. Innovations targeted at catheter related care must address not only hospitalized patients but those in non-acute care facilities, as well as those living their lives with indwelling devices in their homes and in their communities.
PIVC insertion presents risks to patient and staff safety
Peripheral intravenous catheter (PIVC) insertion is the most common invasive procedure performed in hospitals today.1 As many as 80% of hospital inpatients require intravenous access at some point in their stay.2
PIVCs have long been considered as having a lower risk of infection than central lines, but their frequent use and high volume has made them responsible for a greater number of infections.3
Moureau noted how certain peripheral intravenous catheter features can help reduce contamination during insertion. Finger-grips, easy slide catheter advancement tabs, integrated extension sets, and blood control valves can aid the clinician in performing an ANTT without contamination of the catheter or insertion site.
According to Pitts, PIVC insertion presents risks to not only patients but also healthcare providers as needlestick injuries can be sustained during the procedure exposing providers to potentially infectious materials.5
B. Braun offers programs and technologies to help improve safety on both sides of the caregiver/patient equation. Through the company’s Peripheral Advantage Program, real-time observations of healthcare workers are observed while performing PIVC insertions in participating hospitals.
Speaking specifically about ultrasound guided peripheral catheter insertions, Moureau said lack of standardization in the procedure with much variability makes contamination during insertion common. She stated:
“Protective products that work together with catheters for ultrasound guided peripheral catheter insertions help to reduce contamination. Products like sterile barrier dressings that allow separation of the ultrasound and gel reduce contamination from the probe and gel, and sterile probe covers all provide a level of probe and insertion site protection that reduce contamination during the insertion.”
“While nothing can eliminate touch contamination 100% of the time, education and training are also requirements to increase safety and reduce risk to the patient,” Moureau added. “Standardization of procedures leads to greater consistency with steps and safety, reducing contamination and patient risk.”
Scrub the hub compliance requires sustained education, compliance monitoring and supply access
Needleless connectors (NC) used with central venous access devices (CVAD) play an important role in preventing needlestick injuries but may increase the risk for central line-associated bloodstream infections (CLABSI) as microorganisms can collect on the external surface of the NC.6 Disinfection of the NC with an antiseptic scrub (chlorhexidine/alcohol or alcohol) before device access has been shown to reduce infection risk.7
“As facilities continue to strive for zero infections, following all aspects of the bundle has never been more important,” said Amanda Thornton, RN, MSN, CIC, VA-BC, PDI Clinical Science Liaison. “However, studies show that even though this is the case, there continues to be huge variances in the consistency in processes of nurses caring for central lines, with one study showing that 31% of healthcare workers failed to disinfect the needleless access site prior to use,8 and another study showing differences that range from 6% to 51% compliance with scrubbing the hub.”9
The challenge lies in clinician adherence to required scrub and dry times, as revealed by a new human factors analysis presented at the Association for Vascular Access (AVA) 2022 Annual Scientific Meeting. While the critical care nurses studied generally recognized the importance of NC disinfection, they lacked knowledge around antiseptic products’ instructions for use (IFU) and facility specific protocols.
Bedside availability of NC antiseptic products was found to be a strong facilitator of NC disinfection best practices, as Hebden explained:
“There’s absolutely no question that having the supplies right there at the bedside is a facilitator of practice and not having them there is clearly a barrier. I really think we need to get more innovative in how we assist the provider with not only the product availability but also the timing. For example, providing antiseptic pads on a tear off strip hanging from the IV pole, along with a tool for counting down the seconds for scrubbing and dry times.”
The amount of time required per NC access when following best practices for disinfection was another barrier discovered during the research. As Hebden noted, with continued staff shortages, nurses are looking for ways to alleviate their workloads. In some cases, compliance monitoring of NC disinfection has fallen to the wayside in the face of competing priorities.
“This kind of research where the care providers are fitting the practice into their workflow is really critical in terms of adoption and sustainability,” she added. “We need to ensure that whatever we’re asking them to do is scientifically based and that we continue to look for technology or tools that will make it easier and more adaptable to their workflow.”
Thornton explained that by utilizing practices for scrubbing the hub that set nurses up to succeed, patients are protected and CLABSIs are avoided:
“Over the past 15 years, significant evidence continues to emerge that when needleless connectors are scrubbed prior to access using a CHG and alcohol combination versus alcohol alone, CLABSI rates not only drop, but are sustained.10 Part of this is due to the very potent antimicrobial combination, but perhaps the more important piece of the puzzle is the fact that when nurses scrub the hub using CHG and alcohol, they only have to scrub for five seconds to achieve disinfection of the needleless connector which nearly eliminates poor compliance and variances in the scrub the hub practices seen across the nation.”
Safe and effective CVC disinfection for ESRD patients
Among the 600,000+ end stage renal disease (ESRD) patients receive hemodialysis in the U.S., 20% of them receive their chronic therapy via a central venous catheter (CVC).11
“Care of the CVC requires diligent and aseptic practice when accessing the catheter limbs during dialysis therapy and when cleansing the skin at the CVC insertion site,” Padovan continued. “Products used for CVC disinfection and antisepsis should be safe, effective, broad spectrum, fast acting, and most importantly, compatible with the catheter materials.”
Angelini Pharma has a 40-year presence in dialysis and manufactures sodium-hypochlorite products specifically for use on these long-term catheters. Alcavis 50 is an FDA-cleared disinfectant used on catheter limbs before accessing the bloodstream. ExSept Plus in an all-purpose skin, wound and catheter exit site antiseptic.
“As the standard of care in dialysis, this compliment of products works in tandem to decrease the risk of infection in this fragile population,” Padovan added.
PICC protection with the patient in mind
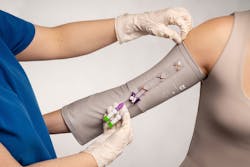
Prolonged maintenance of CVCs, including peripherally inserted central catheters (PICC), is a major risk factor CLABSI, but patients living with these devices don’t always get the information or products they need to prevent risk for contamination. Emily Levy, Mighty Well Co-founder, President and CBO, described her experience:
Recognizing how other patients living with disabilities, chronic conditions, and illnesses were faced with the same challenge, Levy and her two best friends, María del Mar Gómez and Yousef Al-Humaidhi, founded Mighty Well, launched its Friends in the Fight patient community, and developed the company’s “hero product” the PICCPerfect PICC Cover.
PICCPerfect is a patented sleeve constructed of advanced fabric technology featuring antimicrobial, moisture-wicking, and anti-odor properties. Double access points make it easy to access single and double lumens without exposing the PICC insertion site. Patients can choose from 12 color options in seven different sizes.
The company’s motto says it all: “You are not a diagnosis — you’re Mighty Well!”
“Patients are more than a diagnosis, but healthcare providers often look at us as names on a chart and medical device manufacturers typically don’t consider our experience going home with their devices,” said Levy. “I want clinicians, hospital leaders and device companies to think about how we’re their customers, and we’re paying for care. With the trend of more patients receiving home care, I believe the industry must evolve to accommodate our needs.”
A study on PICCPerfect, presented at the 2018 AVA Annual Scientific Meeting, found the use of protective garments increase patients’ quality of life and are an important part of the continuum of care post-PICC placement, especially considering the lack of quality of options in the standard of care. Key findings include:
- 21.2% of clinicians recommend patients wear a cut-off sock to protect a PICC, and 29.4% recommend stockinette
- 67.1% of patients report they never experience pulling on the line when using PICCPerfect, double that of patients using stockinettes (33.8%)
- 54.5% of patients reported that they felt considerably more comfortable when wearing a PICCPerfect vs. using a stockinette provided by the clinician
- 12.8% of patients reported more than one infection while using stockinette. The number is reduced to 4.7% for patients while using PICCPerfect
In 2020, Mighty Well designed, launched, and registered the PICCPerfect Pro (Rx) with the U.S. Food and Drug Administration (FDA) as a Class 1 medical device. It has another level of securement with a velcro wrap, triple “X”-shaped access points, and offers more visibility to the insertion site with airflow perforation. PICCPerfect Pro was created with input from over 250 medical experts, patients and PICC nurses, as well as the learnings from the analysis of the published iPoster at AVA. While patients can purchase the original PICCPerfect directly from Mighty Well’s website using their FSA/HSA dollars, the Pro version requires a doctor’s prescription. PICCPerfect Pro (Rx) is patent pending.
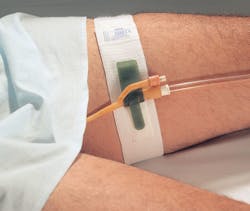
Foley catheter securement for safety
According to the Centers for Disease Control and Prevention (CDC), between 15-25% of hospitalized patients receive urinary (also called Foley) catheters during their hospital stay.12 Traumatic, unintended Foley catheter removal (patient-initiated or accidental), “can cause permanent urologic complications, affect hospital length of stay, decrease patient satisfaction grades, increase catheter-associated urinary tract infections (CAUTI), and lower hospital quality scores.”13
“Dale urinary securement products help prevent this unexpected movement from occurring by way of the design of their products,” Zilaro continued. “All Dale HnP Foley securement products allow for a degree of adjustment through unique latching straps to deter unintended movement and provide both safety and comfort to patients that wear them.”
PICC securement without sutures or adhesives
With CLABSI being a major concern among healthcare organizations, researchers have studied how various PICC line securement methods impact CLABSI rates: adhesive, subcutaneous, tissue adhesives, integrated dressings, and sutures.
An analysis published in the June 2020 American Journal of Infection Control compared outcomes of patients whose PICCs were secured with the SecurAcath Subcutaneous Anchor Securement System (SASS) to those secured with an adhesive device.14
The study found a substantial difference in relative risk among securement devices utilized in their population. The analysis showed those who had an adhesive device had a 288% increase in risk of CLABSI compared to those who had a SecurAcath. The difference in practice demonstrated direct positive impact on patient outcomes when using SecurAcath verses an adhesive securement device.
The design features of the SecurAcath believed to have positively impacted CLABSI rates are improved catheter stability at the insertion site, reduced catheter movement, including migration and dislodgement requiring catheter replacement, and the ability to disinfect skin/insertion site 360°.
Looking ahead
Researchers and manufacturers continue to study and develop new technologies and techniques to improve catheter safety.
Tetra EDTA catheter flush stops microbes in their tracks
“Due to their invasive nature, CVADs are prone to complications such as bacterial biofilm production and colonization, catheter-related bloodstream infection, occlusion, and catheter-related venous thrombosis,” stated a group of Canadian researchers studying the effectiveness of the KiteLock Sterile Locking Solution, a 4% percent tetrasodium ethylenediaminetetraacetic acid (EDTA) fluid, in controlling these types of complications. KiteLock is approved by Health Canada as a catheter locking solution.
They are planning to perform a multi-center, cluster-randomized, crossover trial evaluating the impact of KiteLock on a primary composite outcome of the incidence rate of CLABSI, catheter occlusion leading to removal, and use of alteplase to resolve catheter occlusion compared to the standard of care. The study will be performed at five critical care units.15
“Invading micro-organisms require calcium and/or magnesium for conversion from a planktonic (or traveling phase) to attachment phase. Once attachment is achieved, biofilm and microbial spread begins. If the invading organism cannot attach to the surface of the catheter, there is unlikely to be a proliferation of microbial-biofilm growth and microbial spread.”
Hatton said while specialty coatings can reduce the risk; they have a limited “resistant life” that eventually leaves the catheter vulnerable to microbes. Instead, Hatton points use of a repeatable, liquid application that can “renew the resistant characteristics” as a potential solution.
“A broad-spectrum efficacy range is needed for true protection,” Hatton explained. “When the catheter in use has an option to have the interior of the catheter filled (or rinsed) with a broad-spectrum EDTA solution (as with vascular or urological catheters) the resulting protection can be significant and the resulting prevention of microbes available to ‘spread’ will impact the cross-contamination process.”
“With a broad-spectrum EDTA liquid in use, the prevention of microbial attachment is inherent,” Hatton added. “The Tetra EDTA solution (both 2% and 4%) will also chelate, or grab, the organism’s nuts and bolts (minerals), causing deterioration of the organism. A less than diligent moment by an individual may be offset with a broad-spectrum barrier to provide the insurance needed to prevent microbial transfers. Appropriate formulation details will need to be studied for multiple environments to assure the Tetra EDTA solution will be successful.”
The future of Foley catheter construction
Despite efforts to reduce CAUTIs among patients, they remain the most common nosocomial infection, accounting for 1 million cases per year in U.S. hospitals. The associated costs of preventable CAUTI are estimated to range from $115 million to $1.82 billion annually.16 Duration of catheter placement is a primary risk factor for CAUTI, with some Foley catheters remaining indwelling for up to 30 days.
“Microorganisms can colonize urinary catheters because of contamination during placement, the migration of intestinal microbes to the urinary tract, or the reflux of collected urine into the bladder. Catheter surfaces enable microbial attachment and growth into biofilm and result in bladder tissue invasion and CAUTI. If untreated, CAUTIs can lead to sepsis. The most common remedy for CAUTI requires catheter replacement and antibiotic therapy. Over time, this can promote microbial resistance.”
“Today, urinary catheter manufacturers employ antimicrobial catheter coatings to prevent CAUTIs,” Vachon continued. “Overwhelmingly, the antimicrobial agent employed is silver in its various forms. However, antimicrobial silver catheters have not solved the problem as demonstrated by large randomized clinical trials. Poor outcomes have been attributed to the challenging biological environment and perhaps the inability of coatings to deliver microbicidal doses of silver over 30 days.”
IMSC is developing a unique, next generation antimicrobial catheter that does not use an antimicrobial coating. Instead, IMSC utilizes a proprietary antimicrobial (silver) composite silicone to construct its catheter. The composite silicone (reservoir) remains microbicidal for at least 30 days by virtue of its unique controlled release mechanism. IMSC catheter development has been funded by the Congressionally Directed Medical Research Programs (Peer Reviewed Medical Research Program, W81XWH-16-1-0697 and the CDMRP Spinal Cord Injury Research Program, W81XWH-22-1-0540).
“The IMSC catheter will provide safe, adjunctive protection against infection beyond good standard of care and accepted clean (aseptic) insertion of urinary catheters. The novel IMSC biomaterial is not a replacement for this standard of care,” said Vachon.
References:
1 Helm, R.E., Klausner, J.D., Klemperer, J.D., Flint, L.M. & Huang, E. (2015). Accepted but unacceptable: Peripheral IV catheter failure. Journal of the Infusion Nurses Society, 38(3), 189-203
2 Beecham GB, Tackling G. Peripheral Line Placement. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539795/
3 Mary Duncan, Patricia Warden, Stéphanie F. Bernatchez, Dan Morse, A Bundled Approach to Decrease the Rate of Primary Bloodstream Infections Related to Peripheral Intravenous Catheters, Journal of the Association for Vascular Access, Volume 23, Issue 1, 2018, Pages 15-22, ISSN 1552-8855, https://doi.org/10.1016/j.java.2017.07.004, https://www.sciencedirect.com/science/article/pii/S1552885517300454)
4 Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., Alexander, M. (2021). Infusion Therapy Standards of Practice (8th ed). J Infus Nurs. 44 (1S), S1-S224
5 Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., Alexander, M. (2021). Infusion Therapy Standards of Practice (8th ed). J Infus Nurs. 44 (1S), S1-S224
6 Methods for microbial needleless connector decontamination: A systematic review and meta-analysis, American Journal of Infection Control, February 27, 2019, https://www.ajicjournal.org/article/S0196-6553(19)30006-9/fulltext
7 Martinello R, Hebden J, Drews F, Pegues D. Human factors analysis of the disinfection of central-line needleless connectors. Antimicrob Steward Healthc Epidemiol. 2022 May 16;2(Suppl 1):s32–3. doi: 10.1017/ash.2022.117. PMCID: PMC9614877.
8 Karchmer T, Cook E, Palavecino E, Ohl C, Sherertz R. Needleless valve ports may be associated with a high rate of catheter-related bloodstream infection. Proceedings of the 15th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America: Los Angeles, CA. April 2005
9 Drews FA. An evaluation of methods to reduce I.V. catheter related bloodstream infections. Am J Infect Control. 2013;41(6):S91
10 Amy Paradis RNC, NNP-BC, CNS, Doctors Medical Center, Neonatal Intensive Care Unit, Modesto, CA. “Chlorhexidine Gluconate (CHG): A Key Piece of the BSI Puzzle” Abstract presented at National Advanced Practice Neonatal Nurses Conference in Palm Springs CA, May 2019
11 United States Renal Data System 2020 Annual Data Report
12 Catheter-associated Urinary Tract Infections (CAUTI), CDC, https://www.cdc.gov/hai/ca_uti/uti.html
13 Prevention of Inappropriate Self-Extraction of Foley Catheters, May 29, 2022, https://www.ncbi.nlm.nih.gov/books/NBK482270/
14 M.S. Rowe, K. Arnold, T.R. Spencer, Catheter securement impact on PICC-related CLABSI: A university hospital perspective, American Journal of Infection Control, Volume 48, Issue 12, 2020, Pages 1497-1500, ISSN 0196-6553, https://doi.org/10.1016/j.ajic.2020.06.178.
15 Ornowska M, Wong H, Ouyang Y, Mitra A, White A, Willems S, Wittmann J, Reynolds S. Control of Line Complications with KiteLock (CLiCK) in the critical care unit: study protocol for a multi-center, cluster-randomized, double-blinded, crossover trial investigating the effect of a novel locking fluid on central line complications in the critical care population. Trials. 2022 Aug 30;23(1):719. doi: 10.1186/s13063-022-06671-5. PMID: 36042488; PMCID: PMC9425798.
16 Werneburg GT. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res Rep Urol. 2022 Apr 4;14:109-133. doi: 10.2147/RRU.S273663. PMID: 35402319; PMCID: PMC8992741
The EBP complexities of catharized nursing home patients

Lisa Murtagh
Prior to performing high contact resident care activities (e.g., dressing, bathing/showering, transferring, changing linens/briefs, toileting assistance, device care or use, wound care), the caregiver must don personal protective equipment (PPE), including gloves and a gown, and potentially face protection if there is a risk of splash or spray. After care is completed, they most doff the used PPE and don a new set before caring for the next patient.
Infection preventionist Lisa Murtagh, LPN, IP, who works in a Massachusetts nursing home, says while the EBP are an important step in reducing the transmission of MDROs among residents, they present challenges as well, especially as facilities face ongoing staff shortages. She presents the scenario of patient rehab as an example of the complexity:
“Typically, when rehabbing a patient, even those with a catheter, the physical therapist (PT) and occupational therapist (OT) will ambulate the patient in the hallway. Because the CDC considers this a high-contact activity, under the new EBP guidelines the PT and OT are required to wear gloves and gowns but doing so in a hallway goes against policy. I asked the Massachusetts Department of Public Health about the conflicting requirements, and they told me that we would have to end hallway ambulation for patients where EBP are required.”
Murtagh explains that to perform PT and OT on a catharized patient in the facility’s gym, a caregiver would need to don a gown and gloves, transfer the patient out of their bed into their wheelchair, doff the PPE at the door, push the wheelchair to the gym, don a new set of PPE to transfer and ambulate them, doff the PPE again to transfer the patient back to their room, don yet another set of PPE to transfer them back to their bed, and doff that PPE and don another set before caring for a new patient.
“Not all PPE is made equally,” said Murtagh. “Some of it is easy to get off, others are extremely difficult and when you can’t get what you need you have to order what is available. The next thing you know is you’re trying to get a gown off that won’t untie and end up stepping out of it. It’s a mess. There’s just not enough time in the day.”
References:
1 Implementation of Personal Protective Equipment (PPE) Use in Nursing Homes to Prevent Spread of Multidrug- resistant Organisms (MDROs), CDC, July 12, 2022, https://www.cdc.gov/hai/pdfs/containment/PPE-Nursing-Homes-H.pdf
About the Author
Kara Nadeau
Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.