GAO report: Critical Vaccine Distribution, Supply Chain, Program Integrity, and Other Challenges Require Focused Federal Attention
The U.S. Government Accountability Office (GAO) released its fifth comprehensive report since June 2020 about the implementation of the CARES Act. This report makes 13 new recommendations to improve agencies' public health and economic recovery efforts, including the development of a national testing strategy.
They continued:
We remain deeply troubled by the lack of sufficient federal action on critical gaps identified and by the lack of clear plans to address these gaps. For example, a clear and comprehensive vaccine distribution plan remains a work in progress.
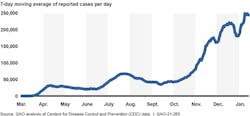
Since November 2020, the number of COVID-19 cases in the U.S. has rapidly increased, further straining healthcare systems across the country. Between Dec. 31, 2020 and Jan. 13, 2021, new reported COVID-19 cases averaged about 225,000 per day—over seven and three times higher than the surges the nation experienced during the spring and summer of 2020, respectively. The country also continues to experience serious economic repercussions and turmoil as a result of the pandemic. As of December 2020, there were more than 10.7 million unemployed individuals, compared to nearly 5.8 million individuals at the beginning of the calendar year. Until the country better contains the spread of the virus, the pandemic will likely remain a significant obstacle to more robust economic activity.
In this report, GAO is making 13 recommendations to federal agencies to improve the ongoing response and recovery efforts in the areas of public health and the economy. As the new Congress and administration establish their policies and priorities for the federal government’s COVID-19 response, GAO urges swift action on these 13 recommendations, as well as on the additional recommendations that GAO has made since June 2020.
As of January 2021, 27 of GAO’s 31 previous recommendations remained unimplemented. GAO remains deeply troubled that agencies have not acted on recommendations to more fully address critical gaps in the medical supply chain. While GAO recognizes federal agencies continue to take some steps, GAO underscores the importance of developing a well-formulated plan to address critical gaps for the remainder of the pandemic, especially in light of the recent surge in cases. In addition, implementation of GAO’s recommendation concerning the importance of clear and comprehensive vaccine distribution and communication plans remains a work in progress. Moreover, slow implementation of GAO’s recommendations relating to program integrity, in particular those made to the Small Business Administration (SBA) and Department of Labor (DOL), creates risk of considerable improper payments, including those related to fraud, and falls far short of transparency and accountability expectations.
GAO’s new recommendations include:
· GAO is recommending that the Department of Health and Human Services (HHS) develop and make publicly available a comprehensive national COVID-19 testing strategy that incorporates all six characteristics of an effective national strategy.
· In September 2020, GAO stressed the importance of having a plan that focused on coordination and communication and recommended that HHS, with the support of the Department of Defense, establish a time frame for documenting and sharing a national plan for distributing and administering COVID-19 vaccine, and among other things, outline an approach for how efforts would be coordinated across federal agencies and nonfederal entities. To date, this recommendation has not been fully implemented. GAO reiterates the importance of doing so.
· To improve the nation’s response and preparedness for pandemics, GAO recommends that HHS establish a process for regularly engaging with Congress and nonfederal stakeholders—including state, local, tribal, and territorial governments and private industry—as the agency refines and implements its supply chain strategy for pandemic preparedness, to include the role of the SNS.
· To help it identify and mitigate vulnerabilities in the U.S. drug supply chain, GAO recommends that the Food and Drug Administration (FDA) ensure drug manufacturing data obtained are complete and accessible, including by working with manufacturers and other federal agencies, such as the Department of Defense and the Department of Veterans Affairs and, if necessary, seek authority to obtain complete and accessible information. HHS neither agreed nor disagreed with this recommendation.
· To improve the federal government’s response to COVID-19 and preparedness for future pandemics, GAO recommends that HHS immediately establish an expert committee comprised of knowledgeable healthcare professionals from the public and private sectors, academia, and nonprofits or use an existing one to systematically review and inform the alignment of ongoing data collection and reporting standards for key health indicators. HHS partially concurred with this recommendation and agreed that it should establish a dedicated working group or other mechanism with a focus on addressing COVID-19 data collection shortcomings.
· GAO recommends that FDA (1) ensure that inspection plans for future fiscal years identify, analyze, and respond to the issues presented by the backlog of inspections that could jeopardize its goal of risk-driven inspections, and (2) fully assess the agency’s alternative inspection tools and consider whether these tools or others could provide the information needed to supplement regular inspection activities or help meet the agency’s drug oversight objectives when inspections are not possible in the future. FDA concurred with both recommendations.
· To ensure consistent tracking and transparency of federal contracting activity related to the pandemic, GAO recommends that HHS accurately report data in the federal procurement database system and provide information that would allow the public to distinguish between spending on other transaction agreements and procurement contracts. HHS concurred with this recommendation.
· To improve its oversight, GAO recommends that OSHA (1) develop a plan, with time frames, to implement the agency’s oversight processes for COVID-19-adapted enforcement methods, and (2) ensure that its data system includes comprehensive information on use of these enforcement methods to inform these processes. The agency neither agreed nor disagreed with these recommendations.
· GAO recommends that OSHA determine what additional data may be needed from employers or other sources to better target the agency’s COVID-19 enforcement efforts. The agency neither agreed nor disagreed with this recommendation.
· GAO recommends the development of a mechanism to track the progress of states, tribes, and territories in meeting established timelines to disburse funds in an expedited and efficient manner. NOAA concurred with this recommendation.
· To better track the recovery of federal funds, GAO recommends that DOL collect data from states on the amount of PUA overpayments recovered. DOL concurred with this recommendation and has taken the first step toward implementing it by issuing new guidance and updated instructions for states to report PUA overpayment recovery data.