The Path Forward: A federal perspective on the COVID-19 response
Dr. Janet Woodcock, Acting Commissioner of the U.S. Food and Drug Administration (FDA or the Agency) testified Monday describing the FDA’s coronavirus disease 2019 (COVID-19) response efforts. "All of our efforts are in close coordination and collaboration with our partners, both within the Department of Health and Human Services (HHS) and across the Federal government, to help ensure the development, authorization, licensure, and availability of critical, safe, and effective medical products to address the COVID-19 public health emergency."
From the beginning of this public health emergency, FDA has taken an active leadership role in the all-of-government response to the COVID-19 pandemic, to ensure the FDA is doing everything possible to protect the American public, help ensure the safety, efficacy, and quality of FDA-regulated medical products, and provide the industries we regulate with the guidance and tools to do the same. They continue to focus on facilitating the development and availability of medical countermeasures to diagnose, treat, and prevent COVID-19, surveilling the medical product and food supply chains for potential shortages or disruptions, and helping to mitigate such impacts, as necessary to protect the public health.
FDA’s Center for Biologics Evaluation and Research (CBER) uses every tool available to help patients access promising biological products while facilitating research to evaluate their safety and efficacy as well as manufacturing efforts.
CBER is working on multiple fronts to address the COVID-19 pandemic, including:
- Expediting clinical trials for vaccines and certain therapeutic biological products that hold promise to prevent or treat COVID-19 by providing timely interactions, scientific advice, and recommendations for specific sponsors, and generally through guidance documents;
- Supporting product development and facilitating the scaling up of manufacturing capacity for high priority products to treat COVID-19;
- Expediting the review of Emergency Use Authorization (EUA) requests and Biologics License Applications (BLAs) for critical medical products to address COVID-19;
- Helping to ensure an adequate and safe blood supply; and
- Providing information to healthcare providers and researchers to help them submit expanded access IND requests to permit the use of investigational products for patients with COVID-19.
Through our transparent scientific review process, FDA has issued EUAs for three COVID-19 vaccines. In doing so, we have relied upon the Agency’s rigorous standards for safety, effectiveness, and manufacturing quality. For the three vaccines authorized to date, our EUA process not only included a thorough evaluation of the data by the Agency’s career staff, but also included input from independent scientific and public health experts through our public advisory committee meetings. Throughout this process, FDA took additional steps to facilitate transparency, such as posting sponsor and FDA briefing documents and key decisional memoranda.
It is also important to highlight that, as part of each EUA, we are requiring the manufacturers and vaccination providers to report serious adverse events, cases of Multisystem Inflammatory Syndrome (MIS), and cases of COVID-19 that result in hospitalization or death to the Vaccine Adverse Event Reporting System (VAERS), a national vaccine safety surveillance program jointly run by FDA and the Centers for Disease Control and Prevention (CDC).
These surveillance efforts have led the Agency to take steps to proactively address emerging safety signals. In April, out of an abundance of caution, FDA and CDC recommended a pause in the use of the Janssen COVID-19 vaccine while we investigated reports of thrombosis with thrombocytopenia syndrome. Later that month, after careful evaluation of the data, FDA announced revisions to the vaccine recipient fact sheet to include information about the risk of thrombosis with thrombocytopenia, and the vaccination provider fact sheet to include a warning about the risk of thrombosis with thrombocytopenia syndrome. We concluded that the available data suggest that the chance of this serious adverse event occurring is very low.
On June 25, FDA announced revisions to the vaccine recipient and caregivers and vaccination provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis and pericarditis following vaccination. The chance of these adverse events occurring following administration of either the Moderna or Pfizer-BioNTech COVID-19 vaccine appears to be very low, but the level of potential risk due to vaccination is still under investigation
On July 12, FDA announced revisions to the vaccine recipient and caregivers and vaccination providers fact sheets for the Janssen COVID-19 vaccine regarding a suggested increased risk of Guillain-Barré syndrome during the 42 days following vaccination. The chance of this occurring following vaccination appears to be very low.
At this time, data are not yet available to make a determination about how long these authorized vaccines will provide protection, nor are we certain that the vaccines prevent transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from person to person.
Finally, manufacturers whose COVID-19 vaccines have been authorized for emergency use are expected to continue their clinical trials in order to obtain additional safety and effectiveness information and pursue licensure (approval).
Medical Devices - The need for medical devices to respond to the COVID-19 pandemic has far exceeded what we experienced in any prior Public Health Emergency (PHE). The first EUAs issued for the COVID-19 PHE were for medical devices, and the volume of EUA requests quickly surpassed (by two orders of magnitude) that of any prior PHE or other situation. Further, the emergency use requests included submissions for devices that CDRH had never received EUA requests for during prior PHEs. This included ventilators and novel devices such as continuous renal replacement therapy devices. Since the start of the pandemic, FDA has issued EUAs or granted full marketing authorization to almost 1,500 medical devices for COVID-19-related uses.
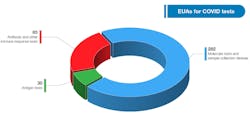
Diagnostic tests are the first line of defense in an outbreak, and FDA plays an important role to ensure they work through EUA review. The EUA pathway expedites access to accurate diagnostic tests during emergencies, when information gaps and false results may adversely affect individual patient care and public health decision making. EUAs enable molecular diagnostic tests to be developed, validated, authorized, and deployed within weeks rather than several months to over a year, as is typical for test development and traditional premarket submissions. As of July 13, 2021, these templates have received 510,725 hits from those visiting FDA’s website, and has authorized 397 tests and sample collection devices for SARS-CoV-2.
FDA has authorized a wide variety of other medical devices for use in combating the pandemic, including a wide range of personal protective equipment (PPE), ventilators, and other therapeutic devices. As of July 13, 2021, FDA has authorized 270 PPE devices including 39 surgical masks, and has authorized 205 filtering facepiece respirators (FFRs), 13 systems for PPE decontamination or bioburden reduction at the time there was a need for these types of devices due to PPE shortages, and 13 EUAs for face shields and other barriers intended to protect the user from bodily fluids, liquid splashes, or potentially infectious materials (see related graphic below). In addition to granting EUAs, FDA has also cleared, through its premarket notification pathway, over 250 PPE 510(k)s.
Inspections, Compliance, and Protecting the Medical Supply Chain - Through continued vigilance, FDA has prevented unsafe and unauthorized pharmaceuticals and other medical products from entering the country. Since March 2020, with the cooperation of and in coordination with CBP, FDA has received and destroyed almost 60,000 products, totaling over 11,093,868 capsules, pieces, and tablets of illegal or unapproved drugs and has refused approximately 94,725 lines of imported violative medical products.