FDA issues update on potential infection risk when using heater-cooler devices
The U.S. Food and Drug Administration (FDA) continues to monitor the risk of Nontuberculous mycobacteria (NTM) infections in patients who have undergone cardiothoracic surgery using heater-cooler devices, and to collaborate with stakeholders including public health partners, manufacturers and experts to evaluate additional strategies to reduce the risk of infection from using these devices during cardiothoracic surgery.
The FDA is providing updated information from our ongoing evaluation and monitoring of actions being taken by heater-cooler manufacturers:
- Two heater-cooler manufacturers (CardioQuip and Gentherm Medical LLC) announced voluntary recalls for updated labeling with interim mitigation strategies to help reduce the risk of NTM infections in patients when using these devices, while these manufacturers complete further testing for cleaning and disinfection validation, and aerosolization.
- Two manufacturers (Terumo and Maquet) announced voluntary recalls to tell facilities to discontinue use of their heater-cooler devices because these manufacturers will not be pursuing a cleaning and disinfection protocol that addresses the risks of NTM infection.
- As previously announced in February 2020, the LivaNova Heater-Cooler System 3T was cleared with changes to help reduce the risk of patient infections, with validated cleaning and disinfection instructions and the 3T Aerosol Collection Set to reduce (but not eliminate) the risk of potential emission of aerosols.
The FDA wants to ensure that healthcare providers and users are aware to discontinue use of the Maquet and Terumo heater-cooler devices, and of the interim mitigations from CardioQuip and Gentherm Medical LLC. The FDA is continuing to work with CardioQuip and Gentherm Medical LLC to ensure they provide test results on cleaning and disinfection validation as well as aerosolization. The FDA will continue to work with manufacturers on strategies to minimize the risk of NTM patient infection.
For specific heater-cooler devices in your facility, review the applicable recall notices from each manufacturer or contact the manufacturer directly for more information.
Healthcare providers and users should review the current recommendations from the FDA for the use of any heater-cooler device to help reduce the risk of NTM infections in patients when using these devices during cardiothoracic surgeries:
- Be aware that heater-cooler devices are important in patient care. In appropriately selected patients, the benefits of temperature control during open chest cardiothoracic procedures generally outweigh the risk of infection transmission associated with the use of these devices.
- Strictly adhere to the cleaning and disinfection instructions provided in the manufacturer's heater-cooler device labeling. Ensure you have the most current version of the manufacturer's instructions for use readily available for staff who interact with these devices.
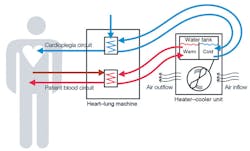
- DO NOT use tap water to rinse, fill, refill, or top-off heater-cooler water tanks since this may introduce NTM organisms. Use only sterile water or water that has been passed through a filter of less than or equal to 0.22 microns. When making ice needed for use in the heater-cooler, use only sterile water or water that has been passed through a filter of less than or equal to 0.22 microns. Deionized water and reverse osmosis-treated water are not recommended because they may promote corrosion of the metal components of the system.
- Always direct and/or channel the heater-cooler device's exhaust vent(s) away from the surgical/sterile fields and toward an operating room exhaust vent during device set-up and surgical procedures as well as after use to mitigate the risk of aerosolized heater-cooler tank water reaching the sterile field.
- Establish regular cleaning, disinfection, and maintenance schedules for heater-cooler devices according to the manufacturer's instructions to minimize the risk of device contamination and patient infection.
- Be aware that not following the heater-cooler manufacturer's cleaning instructions (frequency of cleaning and disinfection, strength of disinfectants used, and so forth) can damage the device.
- Follow a comprehensive quality control program for maintenance, cleaning, and disinfection of heater-cooler devices. This may include written procedures for monitoring adherence to the program and documenting set up, cleaning, and disinfection processes before and after use.
- Use new accessories, tubing, and connectors to prevent possible recontamination when using a different heater-cooler device.
- Be aware that device contamination may also occur from other sources such as environmental contamination or device contact with contaminated accessories.
- Clean, disinfect, and exchange heater-cooler and accessory tubing following manufacturer instructions.
- Follow manufacturer instructions for storage of heater-cooler devices and accessories when not in use, which may include removing all water from the device and tubing.
- Immediately remove from service heater-cooler devices that show discoloration or cloudiness in the fluid lines/circuits. This may indicate contamination. Consult your hospital infection control officials to perform the appropriate follow up measures and report events of device contamination to the manufacturer.
- Consider performing environmental, air, and water sampling and monitoring if heater-cooler contamination is suspected. Environmental monitoring requires specialized expertise and equipment to collect and process samples, which may not be feasible in all facilities.
- Patients suspected of an infection associated with heater-cooler devices or exposed to a contaminated heater-cooler device should be notified and evaluated. Healthcare facilities should follow their internal procedures for notifying and evaluating patients.
Report any issues with heater-cooler devices to the FDA, including if you suspect heater-cooler devices have led to patient infections or any type of contamination of a heater-cooler device.