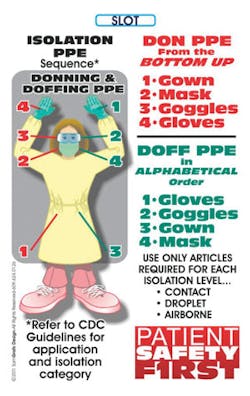
PPE – Prepared for Prevention Every time
Last year, when Ebola made its way into the United States, serious concerns about personal protective equipment (PPE) landed front and center. Healthcare facilities scurried to stock up on what was available while healthcare workers (HCWs) complained of not having access to the right kind or that they lacked sufficient training and knowledge on how to use it properly.
“There were many lessons learned with Ebola about PPE and preparedness,” said Laura Buford, RN, BSN, CIC, APIC Communications Committee Chair. “Mainly, we learned we were not prepared. Now, we know what to do but it has to be practiced. It is very difficult to get in and out of the suits without contaminating yourself. If you don’t practice doing it, it’s pretty definite you’ll have an exposure.”
Poor compliance with donning and doffing of all types of PPE is still a significant — and preventable — problem, as indicated in a study published last December in the American Journal of Infection Control. 1
Researchers found that even when HCWs wore gloves when handling patients withClostridium difficile (C. diff.) they still had spores on their hands because of poor donning and/or doffing. The upside is that after HCWs participated in an educational intervention on correct techniques and disinfection of gloves with bleach, they experienced zero contamination. Finding a more tolerable alternative to bleach would likely improve compliance as well, according to the authors.
“The biggest obstacle to PPE compliance is attitude; healthcare workers have this idea that they’re bulletproof,” suggested Buford, also an Infection Preventionist/Employee Health Nurse at Lakeway Regional Medical Center, Lakeway, TX. “’I don’t need that stuff’ or ‘I’m just going in there for a minute’ or other things are heard frequently as reasons why they aren’t compliant with PPE. Education is done to explain what the PPE is for … but it’s still a battle. Doing compliance rounds on the patients on isolation and watching to see if people are using PPE appropriately have helped to shift attitudes and improve practices.”
Promoting compliance
Finding tools and products that make wearing PPE less onerous might also help in the effort to improve compliance — whether it’s improving access, simplifying steps or providing important reminders.
Healthmark Industries’ Cool Aids line now includes a single-use disposable vest to help manage core body temperature. “When worn under protective barrier attire, they are ideal for use by staff during surgery, reprocessing of devices and other activities because cooling is achieved with the reusable cooling packs rather than with a system of hoses and an external source,” explained Healthmark Marketing Manager Matt Smith. “This innovative design allows for greater freedom of movement without worrying how to get them laundered and returned.”
To encourage proper mask and respirator use, last year APICreleased new educational fliers based on current guidelines that describe the Do’s & Don’ts of using procedure masks and N95 respirators. “The tools were inspired from a conversation our committee was having about people not wearing masks appropriately,” Buford recalled. “We thought it would be a good idea to make an easy-to-use tool for infection preventionists or any others in healthcare facilities of any kind to use for educating on proper usage.” (You can download and print the fliers from the APIC website.2)
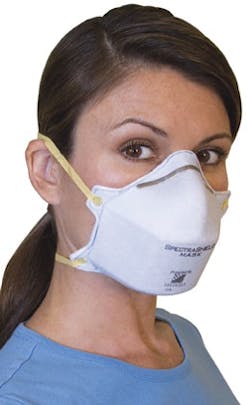
One of the more recent innovations in face/respiratory protection is the SpectraShield 9500 Surgical Mask by Nexera. The product contains silver- and copper-based antimicrobial technology by Sciessent to deliver what they say is the first FDA-approved antimicrobial surgical respiratory mask. According to a press release issued in April, the SpectraShield 9500 kills 99.99 percent of tested bacteria after one hour and will inactivate 99.99 percent of tested influenza viruses after five minutes of contact. “Our SpectraShield 9500 Surgical Mask has proven to be safe for first responders and hospital workers and highly effective in killing airborne pathogens,” said James Morrell, CEO, Nexera. “In fact, it was cleared for eight hours of use, which is more than double the time required by NIOSH and OSHA.”
Badge Buddies by RapidRecalling.com is another helpful product line designed to keep HCWs mindful of the importance of compliance and assist them on the spot with tips on handling a variety of healthcare-related tasks, including PPE use. “Our badges, worn with their ID badges, serve as a ready reference to rapidly recall important information healthcare staff needs to know on a daily basis,” said John Sammons, Partner, Designer, Rapidrecalling.com. “All of our badges are customized to fit the varying needs of our customers. We’re the only badge buddy company out there that we know of that offers truly custom, eye-catching artwork at prices even the smallest facility can afford. Our most popular badges include hospital Emergency Codes with RACE/PASS fire safety procedures, Infection Control, PPE Don/ Doff instructions, Annual Flu Shots, National Patient Safety Goals, and many more.”
Insightful eye defense
During last year’s Association of periOperative Registered Nurses (AORN) Surgical Conference & Expo, TIDI Products conducted an informal postcard survey of clinicians on eye splash incidence and eye protection compliance. The responses were a little unsettling, indicating that while eye splash incidences are a common result of not wearing PPE — 59 percent reported an eye splash or near miss — 36 percent also felt the incidents were underreported. Research shows eye exposure accidents outpace other blood and body fluid exposures.
Evelina Leece, Director of Marketing, Acute Care Consumables,TIDI Products, said there were three top reasons for non-compliance given by survey respondents. They said eye protection is not readily available and on the wall next to the gloves; it required time-consuming assembly and some parts are often missing (they have the lenses but no frames and vice versa); and they don’t feel there is enough time to locate and don the eyewear.
“From tradeshows and survey data [HCWs tell us] eyewear wasn’t easy to find, or it required a technical degree to put the eyewear together,” said Leece. “Staff is using their own prescription eyewear as protective eyewear. We have a clinical study that shows the levels of contamination for reusable eyewear are staggering. Staff members have mentioned at tradeshows that they just don’t have the time to find the eyewear when they are rushing into a patient’s room, or during emergency situations. This is more pervasive in all HCW situations due to availability, but especially clinicians that tend to deal with more emergency situations.”
TIDI’s lightweight Grab ‘n Go preassembled eyewear offers a solution with a convenient dispenser at point of use and wraparound design with optical grade shield. The eyewear also fits over prescription glasses. “A hospital in Southern California reported they successfully avoided 15 eye splashes by wearing TIDI’s Grab ‘n Go eye shields in a three-month period,” said Leece. “TIDI Products offers a process improvement program EyeSplash Zero that assists in reducing eye splashes and improving healthcare worker’s safety.” The TIDIShield EyeSplash Zero Process Improvement Program also provides a calculator that facilities can use to determine the impact eye splash incidents have on worker safety and costs. For example, by avoiding 19.2 eye splash incidents facilities can save an average $113,664 or more per year. (Access the calculator on the TIDI website.3)
Product integrity
As compliance issues remain a challenge, another prominent theme in this year’s conversation involves questions about the quality and safety of PPE itself, particularly gloves and gowns.
The TV news program 60 Minutes caused a stir earlier this year when a whistleblower claimed that Halyard Health sold defective, strikethrough-prone surgical gowns to hospitals during and after the Ebola outbreak.
The former employee said the company’s MICROCOOL surgical gowns, which purport to provide AAMI Level 4 protection, did not meet those industry standards but were “recommended aggressively” anyway. The company vehemently denies the allegations and says the gown “meets ASTM 1671 testing for all critical zones, including gown fabric, tie attachment, and sleeve seams, per its cleared standard (AAMI PB70:2003) and per the current, more demanding, AAMI PB70 standard, which was revised in 2012 and recognized by the Food and Drug Administration (FDA) in 2013.”
Yet, in the wake of such an unsettling report — whether true or false — it’s fair to say that clinicians and supply chain professionals have a right to feel concerned. Healthcare Purchasing News asked Chris Lowery, Senior Vice President and Chief Operating Officer, Halyard Health, to answer a few questions shortly after his appearance on the program.
“The 60 Minutes story essentially retold allegations about our MICROCOOL surgical gowns that have been the subject of an ongoing litigation that began in October 2014,” Lowery said in an email. “We reject those allegations. We understand, however, that the 60 Minutes story may have given the impression that MICROCOOL gowns have been the subject of frequent complaints of strikethrough by healthcare providers and that the gowns pose a safety risk. This is simply not true. MICROCOOL is and has always been safe and effective for its intended use and has an excellent track record in the field.”
The program also featured disturbing photos of surgeons with blood on their skin after doffing the MICROCOOL gowns, something Lowery suggests was potentially due to glove-gown channeling, not defective product.
“Glove-gown channeling is a phenomenon that has been mistaken as strikethrough. Channeling can be observed in deep belly surgical cases, or trauma cases, where the surgeon may reach deep into the incision site and have significant exposure to bodily fluids around the glove-gown sleeve interface,” Lowery said. “Glove-gown channeling occurs when bodily fluids make their way between the surgical glove and gown sleeve as a result of a gap between the two. This causes fluid to get into the channel (ridges or folds) of gown sleeves, but the fluid does not pass through the fabric of the gown. Strikethrough, though, is when fluid passes directly through the gown, compromising the barrier. With MICROCOOL, reports of strikethrough are exceedingly rare with less than one complaint per million gowns sold.”
In December, the FDA issued the final version of its guidance document, “Premarket Notification Requirements Concerning Gowns Intended for Use in Health Care Settings.”4 The document is intended to clarify and describe the premarket regulatory requirements for gowns regulated under 21 CFR 878.4040 and the performance testing needed to support liquid barrier claims for gowns. Gown manufacturers claiming high level protection of its products will need to provide premarket clearance and proof/data that they do in fact live up to barrier protection claims per ASTM/AAMI standards or an equivalent.
“Sample labeling should also be submitted; the provided labeling should clearly identify the level of liquid barrier protection claimed,” said Scientific Reviewer Lauren Lilly during an FDA Webinar earlier this year. “Prior to the existence of ANSI/AAMI PB70, moderate or high barrier protection claims included, but were not limited to, wording such as prevents strikethrough; high fluid protection; impervious; fluid-proof; fluid-resistant; highest level of protection; and impermeable. Because of the lack of specificity with respect to performance characteristics and test methods, FDA now discourages the use of such language, and no longer clears products with such labeling claims. Instead, manufacturers are encouraged to specify the level of liquid barrier protection as per ANSI/AAMI PB70 on all labeling. Labeling should also include the directions for use, the indications for use and, if applicable, validated reprocessing instructions.”
Buford says the FDA’s guidance is a “great idea” and that manufacturers should be held accountable for validating claims. “If a company can’t give you a satisfactory response, I’d suggest looking for a different product,” she said.
Gloves point in new directions
Moving on to the glove front, the FDA announced another proposal earlier this year to ban most powdered gloves and absorbable lubricating powder because they can cause respiratory allergic reactions and other potentially serious adverse events, including severe airway and wound inflammation and post-surgical adhesions. “These side effects have been attributed to the use of glove powder with all types of gloves,” stated the FDA in a March 21 press release.
Edmund S. Tai, Assistant Vice President, Tronex Healthcare, says the powder debate has been going on for more than 20 years and that most recently the focus has been not only on powder but the quality of powder used. “There have been a number of incidents within the healthcare industry where the use of inferior-quality powder by manufacturers has been brought into focus with liability cases,” Tai said. “While Tronex Healthcare utilizes only highly regulated powder by featuring U.S. Pharmacopeia (USP) grade cornstarch, there are others in the industry who have not upheld such high quality standards. In the early stages of disposable glove manufacturing, powder eased the effectiveness of donning, so gloves would not break from the force used to quickly don them, especially with damp hands. Tronex was at the forefront of the industry’s product advances in subsequent years, developing highly evolved material formulations and implementing new manufacturing technologies, both of which ultimately led to powder-free exam glove options.”
Tronex powder-free exam gloves come in a variety of styles and material blends and innovative finishing processes that mimic powder and make donning easy. Tai says the #3166 Series Latex Powder-Free, Fully Textured Exam Gloves are crafted from an innovative natural rubber latex material formulation for superior elasticity and barrier protection and feature a smooth interior polymer coating to ease wet and dry donning. Its NEW AGE Powder-Free Exam Glove has a unique stretch-vinyl compound that provides material memory for a superior fit, stretch, and high tensile strength. Tai says these products also deliver a favorable performance-to-price ratio. “With all of the advancements in exam glove materials and technologies available today, there are a wide range of excellent powder-free alternatives available for this small percentage of users to transition to in eliminating powdered exam gloves entirely.”
Todd Karas, Senior Product Manager, Surgical Gloves, Cardinal Health, says its glove customers started transitioning from powder more than a year ago and that glove selection is influenced primarily by industry recommendations and wearer habits. “Some clinicians have worn the same gloves for many years and simply are not familiar with new advancements in glove coatings, infection prevention performance, fit and feel,” Karas said. “Ensuring that healthcare providers are using the right glove for the right reason is crucial for infection prevention.”
Nonetheless, for the few holdouts, a Cardinal Health infographic illustrating why making the switch is important also suggests change can be hard. “When a surgeon gets used to wearing a glove, and they like the way the glove performs (fit, feel and grip), they may not want to change to something new,” Karas said. “If they haven’t personally experienced a reaction to powder, they may know about the potential risks, but not be too concerned personally. After clinicians become educated about powder and the potential risks, and have a chance to experience and try powder-free alternatives, making the change usually isn’t difficult.
“Powder-free glove manufacturing technology creates more versatility to improve performance, such as the ability to meet specific surgical needs with stronger thickness and grip,” he continued. “In addition, modern double-gloving with a colored underglove helps clinicians quickly identify a sharps breach and minimize exposure time. Cardinal Health offers two blue undergloves specifically designed for double-gloving, so using a powdered glove on top is no longer needed.”
Judith Seltzer, MS, BSN, RN, CNOR, Clinical Director, National Accounts, U.S. Surgical Division, Mölnlycke Health Care, suggests the ban isn’t likely to impact very many facilities as most have already stopped using powder. “The powdered glove segment has been declining at a rate of 15 percent year over year while powder-free synthetic surgical gloves are continuing to grow at a rate of 12 percent year over year,”5 Seltzer said. “In today’s healthcare arena, hospitals are incentivized to reduce the risk of hospital-acquired infections (HAIs). Surgical site infections are the cause of nearly one-third of all HAI infections.6 Maintaining powdered gloves contributes to an increased risk of surgical site infections for patients.”
Stellar performance, protection and comfort are equally important in a powder-free glove and Seltzer says Mölnlycke’s Biogel glove, the first to feature a polymer coating with hydrophilic properties, delivers on all three. “Every glove is air-inflation tested for quality and safety, leading to a reduced chance of a hole with an industry-leading AQL level of 0.65 compared to the FDA requirement of 1.5,” she said. “Biogel is a pioneer in developing barrier protection innovations, including double gloving systems that enable an extra layer of protection. In 1993, Biogel introduced a groundbreaking puncture indication system to the double gloving offer, which features a clear, fast and large colored puncture indicator when the outer glove is punctured.”
Seltzer says puncture wounds go undetected as much as 90 percent of the time during surgical procedures.7 “Biogel Indicator Undergloves are engineered to provide the optimum level of contrast with Biogel Overgloves. If the top glove is punctured, fluid penetrates between the two gloves, and a dark patch alerts the wearer to the puncture,” she explained. “This visual indication is even faster with our new thinner glove additions to the product line, Biogel PI Micro and Biogel PI Micro Indicator Underglove.”
Halyard Health also supports the ban on powder and offers powder-free nitrile exam gloves which are designed with the caregiver in mind and are manufactured using proprietary nitrile technology, according to Laurie Clark, BS, MT, (ASCP), Senior Manager, Medical Sciences and Clinical Education, Halyard Health.
“This patented process affords Halyard gloves increased tensile strength and a reduction in glove thickness, resulting in added comfort while providing protection from exposure to bloodborne pathogens and other infectious materials,”8 Clark said. “In a highly competitive market such as exam gloves, quality can be a significant point of differentiation. In order to deliver the highest quality possible, Halyard owns its own manufacturing facility in Thailand and requires the AQL specifications for all of its exam gloves to exceed ASTM standards. Moreover, by offering a portfolio of powder-free nitrile exam gloves at easy-to-understand graduated levels of protection, Halyard makes it easy to choose the right glove for the right task.”
Ansell’s glove portfolio, which includes two new nicely priced styles — the ENCORE Perry Style 42 PF and ENCORE Sensi-Touch PF — also provides a number of unique features. “Our curved anatomical formers create a glove with a more natural fit, decreasing the probability of hand fatigue,” said Pam Werner, MBA, BSN, RN, CNOR, Clinical Consultant, Medical Solutions Global Business Unit, Ansell. “Grip choices allow for secure grip and our Surefit Technology, a cuff enhancement feature to prevent cuff roll down during a procedure, helps prevent unintentional contamination at the sterile field. Our Derma Shield inner polymer coating enhances damp-hand donning.
“Rigorous testing to meet quality standards, from both a regulatory and manufacturer perspective, as well as internal testing and control measures, help assure the end user that the glove is providing superior barrier protection,” continued Werner.
Ansell also introduced its SMART Pack packaging technology, a customizable dispenser that gives users easy access to gloves with two opening choices and easier product identification. It uses 45 percent less shelf space and reduces packaging waste and CO2 emissions.
“The bottom line is that end users want to be protected from blood and blood borne pathogens while still being able to function effectively in the healthcare environment,” Werner said.
References
1. Tomas, E., Sunkesula, V., et al, “An intervention to reduce health care personnel hand contamination during care of patients with Clostridium difficile infection,” American Journal of Infection Control, Vol. 43, issue 12.http://www.ajicjournal.org/article/S0196-6553(15)00768-3/abstract?cc=y=.
2. New “Do’s and Don’ts” of procedure masks and N95 respirators fliers, APIC, November, 10, 2013. http://www.apic.org/For-Media/Announcements/Article?id=93e57b8e-8405-4c7e-ab10-c61099a51402.
3. TIDIShield EyeSplash Zero Process Improvement Program,http://go.tidiproducts.com/tidi-eyesplash-zero-soft-cost-calculator.
4. U.S. Department of Health and Human Services, Food and Drug Administration, “Guidance for Industry and Food and Drug Administration Staff.”http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm452804.pdf.
5. GHX Q12016 Surgical Glove Market Data.
6. CDC Surgical Site Infection (SSI) Event. Procedure-associated Module SSI. January 2014. Available at:http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
7. Timler D, Kusinski M, Iltchev P, et al. “Glove failure in elective thyroid surgery. A prospective randomized study.” International Journal of Occupational Medicine and Environmental Health.
8. Kimberly-Clark Health Care Press Release. “Kimberly-Clark Granted U.S. Patents for Nitrile Exam Glove Technology.” April 26, 2013.
About the Author

Valerie J. Dimond
Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.

.png?auto=format,compress&fit=max&q=45?w=250&width=250)
.png?auto=format,compress&fit=max&q=45?w=250&width=250)