PPE is smarter than ever. How smart are you?
Having to wear so many types of personal protective equipment (PPE) — some in heavy, uncomfortable layers — is probably one of the least favorite parts of a healthcare worker’s (HCW’s) job. Yet it’s imperative they do so because without it, people can get pretty sick, even die.
Fortunately, new technologies are making PPE easier and more tolerable to wear. The not-so-good news is that education about how to choose and properly wear it is still lacking in many facilities. Zooming in on some of the most widely-used PPE, Healthcare Purchasing News explored these and other issues with association professionals, nurses, and vendors to get their take on the matter.
Wanted: A glove to love
To do their jobs successfully, surgeons and other clinicians must wear gloves that not only protect from injury and infection, but they as almost invisible. In fact, in many cases, nothing less will do.
“Glove selection is serious business; if a glove does not provide for both considerations, it is not doing its job,” said Latisha Richardson, MSN, BSN, RN, Clinical Consultant, Medical Solutions, Global Business Unit, Ansell. “The gloves need to fit like a ‘second skin’ for the surgeons that are performing delicate microsurgical procedures; and be flexible enough to touch the tiny hair-like vessels of a premature infant’s heart, in addition to having the durability to withstand the rigor of the chisels and blades during a total joint replacement. All while providing a barrier between the healthcare worker and the patient.”
The biggest complaints when it comes to wearing gloves, says Richardson, involve strength and tear resistance, dexterity and tactile sensation and allergic reactions.
Ansell’s new GAMMEX PI Hybrid features a patent-pending Hybrid Technology, made from a 50/50 blend of polyisoprene and neoprene. The non-latex polymers are interlaced into a crosslink pattern to provide latex-like PI comfort and the high strength of a neoprene glove. For effective breach detection, the semi-transparent GAMMEX PI Hybrid also makes an ideal outer glove when double gloving.
“Now, HCWs no longer must choose between comfort and protection when choosing a glove,” Richardson enthused. “Utilizing research and innovative material blends, new glove technologies are available that provide for comfortable hand barrier protection without sacrificing the much-needed shield against microbial pathogens.”
Talk to the hand, chemical man
According to the Centers for Disease Control and Prevention (CDC), 8 million healthcare workers are potentially exposed to hazardous drugs, including pharmacists, nurses, physicians, OR clinicians, environmental services workers, and others. Exposure to hazardous drugs can lead to a variety of skin and other serious health problems.1
Angela O’Neill, Director of Marketing and Contracts, Acute Care Pharmaceuticals, says her company provides gloves specifically designed to protect hospital pharmacists from hazardous drugs that they commonly encounter.
“The Pharma-Glove has been tested to adhere to or exceed the USP<800> standards2 in the pharmacy setting,” explained O’Neill. “The Pharma-Glove has been permeation tested (ASTM D6978) against the breakthrough of various chemotherapy drugs and IPA as required.”
Scott Stefan, Sales and Product Specialist at Acute Care Pharmaceuticals, noted that the gloves are also designed to address a common complaint: reduced dexterity and slickness when compounding medications.
“The Pharma-Glove is designed with micro textured finger tips and manufactured to be a little thinner than most of the competition to increase dexterity and elasticity while maintaining maximum protection,” Stefan asserted. “Our special construction allows thinner glove material without compromising strength or breakthrough, easily ensuring you can double glove as per USP <800> requirements.”
Additional features, according to Director of Inventory and Purchasing, Dennis McGowan, include a Nitrile glove that is latex-free with 12-inch cuffs, individual, paperless packaging to meet USP<797> and <800> compliance and they’re powder-less. As of January of this year the Food and Drug Administration (FDA) implemented a ban on powdered gloves as well as the use of absorbable powder to lubricate surgical gloves.3
In-your-face protection
Surgical mask development has also come a long way, says Mike Bowen, Executive Vice President of Prestige Ameritech, the largest domestic surgical mask manufacturer in the U.S.
“Several decades of continuous product development in the mask industry has resulted in softer, more breathable fabric and filter materials that more efficiently block bacteria, particles and fluids,” said Bowen. “Today, thanks to the availability of improved nonwoven materials, few masks contain the heat-trapping perforated films of yesteryear. There’s an old saying in the surgical mask making industry: ‘The three things that mask-wearers want most are comfort, comfort and comfort.’ FDA regulations require that masks meet filtration and fluid resistance standards. Clinicians wear masks every day, so they want their masks to be comfortable. Therefore, we use the best US-made materials available, we slit these materials in-house to control their cleanliness, and we manufacture and package everything in our factory near Fort Worth, TX.”
The ties and bindings on the Prestige Ameritech surgical masks are made of polyester-spunlace, which Bowen says is the same silky-soft material that disposable, hospital baby shirts are made of, providing added comfort that clinicians appreciate.
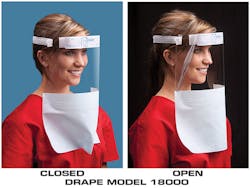
But when it comes to the face shield, there may be more work to be done on many models, according to Roger Machson, President, Onyx Medical Inc. Fogging, discomfort, and no room to wear glasses or goggles are common complaints.
“Protection, comfort and excellent antifogging are the hallmarks of the Drape shields,” said Machson about the Drape shield line from Onyx’s Face-It division.
“The Face-It shields are wonderfully anti-fogged and will not fog up on the wearer. The foam head piece is designed to force the foam into a flat plane in front of the eyes, providing minimal distortion through already crystal clear plastic [and] our head piece foam design holds the foam far enough off the forehead for comfortable wearing of goggles or glasses.”
With a patent pending, the Drape shield provides a 7 ml plastic, 9 x 13 inch full-face barrier that protects ear to ear, forehead, and below the chin. A medical grade foam on the upper side of the shield molds against the forehead to block blood splash from traveling over the top of the shield. Other features include an 8 x 13 inch, AAMI level 3-barrier-fabric on the lower edge of the shield for added splash protection and Velcro-like fasteners to easily cup the fabric under the chin.
“The added protection is significant,” said Machson. “We offer a second version called the Drape Wide which features a 13 x 13 inch, AAMI level 3-barrier-fabric that extends 11 inches below the plastic and is long enough to tuck inside a gown for further protection.”
Gowns that don’t let you down
Gowns are no different. As with any type of PPE, when selecting a surgical or isolation gown, clinicians want equal comfort, function and reliability.
“The material properties and the construction of seams, ties, etc. are important to insure adequate protective qualities,” said Barbara Benson, Product Manager, Protective Apparel, Precept Medical. “Different design features such as, over-the-head, open-back, sizing, and the weight of the material help deliver the comfort, compliance, and other in-use properties.”
Benson said Precept’s PPE gowns have a patent pending for its tear-away, over-the-head full coverage gown, which provides 360-degree coverage. “Also, Precept Medical gowns have more generous sizing proportions, and our wrist area has elastic to help secure the sleeves within the gloves.”
Although clinicians want and deserve to have everything they could possibly need in a surgical gown, it doesn’t always work out that way.
“Most often, clinicians complain about the restrictiveness and overheating tendencies of surgical gowns; surgical procedures can be very physical and time extensive,” said Alex Hodges, General Manager, Surgical and Infection Prevention, Halyard Health. “A gown that is breathable and enables a broad range of motion is the key to addressing these issues.”
Hodges says Halyard’s AERO CHROME Breathable Performance Surgical Gowns’ stretchable, lightweight fabric provides breathability and comfort without sacrificing performance. The gowns have a V-neck design to help prevent gaping, proven fluid and microbial barrier protection, have the “highest rating against ignition from surgical lasers and other heat sources” and minimal lint shed to help reduce the risk of infection.
“Halyard’s AERO CHROME Surgical Gown is FDA approved for AAMI Level 4, the highest level of fluid and microbial protection in the critical zones as defined by the AAMI PB70:2012 standard for liquid barrier performance of protective apparel,” Hodges added. “This protection level provides additional protection from blood-borne pathogens in the critical zones,5 which are defined as those areas where direct contact with fluid is most likely to occur during surgical procedures.”
The gowns are a smart investment as well, according to Hodges, who says an analysis of internal sales data shows facilities that stock the gowns can achieve up to a 40 percent SKU reduction.
“Halyard arrived at 40 percent by calculating how many different but overlapping types of surgical gowns most facilities stock, which could be replaced with Halyard’s two-gown system of AERO BLUE and AERO CHROME gowns,” he said. “In most accounts this would reduce the number of SKU’s in inventory by 40 percent or more while providing AAMI Level 3 and AAMI Level 4 coverage for 80 percent or more of surgical procedures.”
They’re also convenient and color-coded for easy identification. “Clinicians are busy and can’t afford to spend valuable time selecting a surgical gown with the right level of protection for their procedures,” Hodges concluded. “The gowns’ unique colors quickly show clinicians what level of protection they need, and take the guesswork out of gown selection.”
The education situation
Making PPE selection quick and easy is certainly a good idea but should clinicians, as well as those who make PPE purchasing decisions, know what those protection level designations actually mean?
“[The] majority has no idea,” said Vicki G. Allen, MSN, RN, CIC, FAPIC, Chair of the Communications Committee for Association for Professionals in Infection Control and Epidemiology (APIC). Allen is also the Infection Prevention Director at CaroMont Regional Medical Center in Gastonia, NC. “Nursing schools teach the very basics about PPE — what it is and when it’s used — extremely basic! Likely, most of this teaching occurs on the job and mostly during orientation in regards to the specific types of isolation, which is brief and not detailed. Organization signage is usually more detailed and specific to the type of isolation and the PPE required. The education of these topics is extremely varied.”
Judi Coyne, MBA, MA, CGMP, a Health Communication Specialist at the CDC’s National Institute for Occupational Safety (NIOSH), echoes the sentiment. “Yes, I see gaps in the knowledge all the time,” Coyne said, noting that it’s not only gowns and gloves but there’s an inability to differentiate between other types of PPE too. “[Some have] no idea about respiratory protection, understanding of using NIOSH-certified products, the differences between N95 respirators and surgical masks.”
That’s a problem because the risk of using a respirator that isn’t NIOSH-approved means it may not work as it should.
“When a respirator — anything from a filtering face piece to a self-contained breathing apparatus — is submitted to NIOSH for approval, the respirator is subjected to the tests outlined in 42 CFR Part 84 to ensure the product meets the standards,” explained Coyne. “If it doesn’t meet any of the standards, it cannot be labeled as NIOSH-approved. In addition to the initial certification process, NIOSH follows up with product audits. We purchase products from the open market to verify that they are still performing to the standard. NIOSH also visits the manufacturing plants on a routine basis. Again, to ensure everything is still performing to the standard.”
Coyne says the CDC has good information to help facilities become better educated on differentiating NIOSH-approved respirators from counterfeit ones that might appear to be NIOSH-approved.6
Benson, Precept, suggests that more infection preventionists know what ANSI/AAMI standards are compared to caregivers. “The end-users or healthcare workers at the bedside may lack this knowledge and depend upon their Epidemiology/Infection Control department to interpret the standards for protection needed; and to make the decisions for the hospital or facility related to the PPE needed in various departments related to risk of exposure,” said Benson. “Annual safety training in infection control/prevention practices is one way to educate the healthcare worker.”
If Allen’s experience working in hospitals, teaching at a BSN nursing college and still communicating with fellow instructors tells a PPE story, it’s that most healthcare institutions aren’t educating staff on the full scope of PPE. “Teaching about AAMI is virtually non-existent in nursing schools and on the job,” said Allen. “Outside of infection control, employee/occupational health, safety departments and maybe human resources, I would be very surprised if the ‘average’ HCW had ever heard the word NIOSH — it just isn’t taught.”
“One of the issues is the conflicting information that is out there,” suggested Coyne. “There is not a central location for all of the information in easy-to-understand terms. CDC did put out guidance and some videos which were helpful.” NIOSH also offers web-based resources for healthcare PPE
When donning and doffing seems daunting
There’s also a very a specific way to wear and take off PPE to avoid the spread of infection — and there are valid guidelines for getting it right. Yet, compliance remains a challenge for some. Studies, including one featured a couple years ago in the American Journal of Infection Control indicate that many HCWs are not donning and/or doffing PPE according to CDC guidelines. And as the Ebola outbreak showed, many clinicians felt discouraged because either they didn’t have the appropriate PPE on hand, or did not receive adequate training for its use.
So, how should healthcare leaders respond to ensure these problems don’t reoccur?
“By incorporating personnel competency and compliance as related to organization job-specific policies and procedures [and] ensuring routine training when problems are identified,” said Allen. “Routine surveillance rounds by infection prevention and control staff followed by feedback and education are an effective way to ensure staff is compliant and understand the rationale and protocols for donning and doffing PPE.
“My hospital has included PPE donning and doffing competencies in with annual department training,” Allen continued. “Also, we have incorporated extended PPE donning and doffing for first responders, primarily emergency room and critical care staff, in the annual hazard training. The competency includes a required demonstration for the donning and doffing protocol per our organization policy.”
Allen says the hospital infection control sheet from the Centers for Medicare and Medicaid Services (CMS)7 is a good resource organizations can use to ensure staff competence and readiness. “Feedback and audits is a great way to assess staff performance and understanding,” she added, noting that staff turnover and changing job duties make it even more imperative to conduct regular education and training.
Visit www.hpnonline.com/1708-OR-PPE-LISTINGS.pdf for a listing of vendors by product category.
References
1. https://www.cdc.gov/niosh/topics/hazdrug/default.html
2. The U.S. Pharmacopeial Convention (USP)‘s Chapter <800> was written to protect all workers, patients and the general public who may be accessing facilities where hazardous drugs (HDs) are prepared.
4. https://www.ecri.org/Resources/Whitepapers_and_reports/Haz17.pdf.
5. Meets EN13795 High Performance and AAMI PB70: 2012 Level 4.
6. https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/respsource2.html#advisory.
About the Author

Valerie J. Dimond
Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.