Changing payment models, the digitization of healthcare and the need to deliver higher quality care at a lower cost are driving significant change in how healthcare is delivered. Technologies have emerged to support today’s data-driven, value-based care environment. The intensive care unit (ICU), which accounts for 13 percent of hospitals’ costs,1 is a target area for new interventions that can help clinicians detect issues sooner, intervene more effectively and reduce length of stay.
HPN talks with the experts about trends in critical care medicine and new products designed to support healthcare’s changing needs.
Early detection of complications
The combination of acute renal failure and sepsis is associated with a 70 percent mortality rate,2 therefore early detection and management of these complications is key to saving lives. But invasive monitoring for these conditions can require that patients be sedated, which can increase the risk for further complications.3 To overcome these challenges, manufacturers like Deltex Medical have developed advanced non-invasive monitoring technologies that enable clinicians to identify the warning signs sooner.
“There are two main trends impacting our haemodynamic monitoring system offering to ICUs,” said Deltex Medical Managing Director Andy Mears. “Firstly there is a move towards keeping patients awake rather than sedated. This is driving demand for non-invasive haemodynamic monitoring. Secondly there is growing demand for monitors that help the early identification and treatment of sepsis and acute kidney injury (AKI). This is driving demand for high end technologies that can be relied upon to detect small ‘early warning’ changes and then guide complex interventions involving both intravenous fluids and vasoactive drugs.”
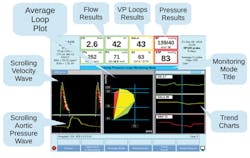
Deltex recently introduced TrueVue Impedance (FDA 510K submitted) to its TrueVue monitoring platform: this is a non-invasive, state of the art High Definition Impedance Cardiography suitable for use on awake patients even when they are moving about. In March 2018, Deltex also introduced its new TrueVue Loops display, which is the first technology to display both central blood flow and pressure beat-to-beat in real time giving clinicians a complete picture of each of the three components of haemodynamics: flow, pressure and resistance. According to the company, this is expected to be a major advance in the prevention, early detection and safe treatment of both sepsis and AKI.
Addressing a sepsis source: In-dwelling urinary catheters
According to the U.S. Centers for Disease Control and Prevention (CDC), urinary tract infections are the most common type of healthcare-associated infection reported to the National Healthcare Safety Network (NHSN), and among UTIs, approximately 75 percent are associated with a urinary catheter.4
“CMS penalties and public sharing of hospital infection rates are causing pressure on ICUs,” said Gregory D. Wiita, President and CEO, Poiesis. “Now healthcare and non-healthcare providers have access to how a hospital is doing respectful to infection rates. An exciting though early use of patient data to predict possible sepsis infection events is being piloted. Instead of relying on equipment EMR data is being employed; hopefully, to identify patients who due to various health issues may become septic. Close to 100,000 lives are lost each year to sepsis related events. Early detection affords patients the best outcomes. In this day and age of massive data sharing it is comforting to know there are positive usages of our data.”
Poiesis released the Duette Dual Balloon indwelling urethral catheter to replace the 80-year-old Foley catheter. The Foley is confirmed to cause wound sites in the bladder, which can lead to sepsis.5 In studies the Duette demonstrated an 80 percent reduction of catheter-associated urinary tract infections (CAUTI).6,7 Now a retrospective review by a level-one trauma facility of 26,000 patients (via EMRs) has reported a 13:1 ratio in favor of Duette technology.8 This added technology provides another lever facilities can use to fight CAUTI. A CAUTI diagnosis carries a 17 percent risk of sepsis and 10 percent of these patients will not survive the event.9 Duette can help to reduce sepsis related deaths in ICUs.
IV infiltration: Preventing liquid invasion
Every year in the United States, millions of adult and pediatric critical care patients receive medications and fluids administered through intravenous therapy (IV) infusion therapy, but according to a recent study, more than 20 percent of IVs fail because of infiltration.10
“Adult and pediatric patients in critical care departments are particularly vulnerable to complications because of the complex nature of their illnesses and they are often times sedated or unable to communicate. We’re hearing from many hospitals about efforts to reduce the severity of outcomes associated with hospital-acquired conditions (HACs) by integrating new technologies into existing protocols,” said Scott Hensley, Vice President of Sales and Business Development, ivWatch. “An adverse event from a HAC, like IV infiltration in the ICU, can negatively impact the patient-provider relationship or carry legal risk for healthcare facilities.”
ivWatch has introduced a new product category — a monitored PIV. The ivWatch Model 400 is a continuous monitoring device that uses a sensor to measure the optical density of the tissue near the IV site to detect PIV infiltrations within a low volume of fluid. Immediate or early warning of infiltrations reduces the risk of patient injury.
“Because the device is always watching the IV site, it gives critical care clinicians a less intrusive alternative to a peripherally inserted central line, and significantly improves the security of a standard unmonitored PIV,” Hensley added.
Temperature management
Patients in the ICU often experience changes in body temperature related to their conditions or caused by interventions. Hypothermia, low core body temperature, and hyperthermia, elevated body temperature, can put patients at risk for complications such as sepsis, and increased mortality rates.
“Patients in the ICU are highly susceptible to changes in their body temperature related to their diagnosis, therapies administered and environment,” said Jenna Lindsay, Technical Service Specialist, 3M Infection Prevention Division. “It’s important to monitor ICU patients’ core temperature closely to track responses to therapies, identify early signs of an infection and to ensure a patient is normothermic. Today there are a wide variety of temperature monitoring devices in use, which can lead to inconsistency with site of measurement, user technique and monitoring frequency. These variations can impact the reliability of accurate temperature readouts, which ultimately can influence important clinical intervention decisions.”
“Non-invasive temperature monitoring modalities have limited reliability to get an accurate core temperature measurement,” she added. “While accurate, invasive modalities have contraindications for ICU patients and can present infection risks, such as catheter associated urinary tract infections (CAUTI).11 This has created a need to use non-invasive patient temperature monitoring methods that provide continuous, accurate, core body temperature measurements.”
The 3M Bair Hugger Temperature Monitoring System is a non-invasive, easy-to-use, accurate temperature monitoring system that continuously measures patients’ core body temperature. The system helps clinicians effectively monitor and manage patient temperature to help improve patient outcomes. The system’s disposable sensor is placed on the patient’s forehead helping reduce temperature variation associated with clinician technique and using multiple device types. The control unit continuously displays core body temperature, trends two hours of patient temperature data and can be connected to the patient monitor by a simple cable connection.
Data-driven care
Hospitals and clinicians are increasingly turning to data in an effort to provide effective interventions that deliver optimal patient outcomes. In the highly demanding ICU environment, clinicians need monitoring technology that is intuitive to use and delivers real-time, actionable information on a patient’s health status.
“Trends in the ICU are focused around improving patient and caregiver safety, data acquisition to guide early decision making for health care providers, protocolized ventilator weaning, use of non-invasive ventilation and high—flow 02 therapy, early mobilization, and reducing overall costs,” said Edwin Coombs, MA RRT, NPS, ACCS, FAARC, Director of Marketing, Intensive Care, Dräger. “These trends are being driven by many different factors including the realization of deaths due to medical errors, the age of big data, reducing length of time on mechanical ventilation or avoiding intubation, identifying recovery time from ICU acquired weakness, and overall changes to healthcare reimbursement schedules.”
Dräger’s Infinity V500 has been designed with these issues in mind. Early warning messages, graphical lung displays, and contextual help options provide clinicians key information that is easily identifiable to guide care. The company’s SmartCare feature provides an automated weaning option that has been shown to reduce average time on the ventilator by 33 percent as compared to conventional weaning methods. Additionally, the V500 is a multi-purpose device that can provide invasive, non-invasive and high-flow 02 therapy within one device, thereby reducing costs and improving workflow at the bedside.
The transportability of the V500 can aid mechanically ventilated patients in early ambulation and support early weaning practices through a variety of options depending on clinical preference. Lastly, the V500 has streamlined preventative maintenance resulting in a low cost of ownership and uses very few proprietary accessories to facilitate a low operating cost per patient.
Maintaining ICU assets
Hospitals invest significant dollars in the technologies required to manage and treat critical ill patients. Therefore it is important that they effectively maintain their equipment assets to keep them in good working order and available when needed. While in-hospital clinical engineering departments can play an important role in servicing medical equipment, Roy Lobb, Director of Product and Service Solutions for PartsSource, reports a growing trend for hospitals to outsource repairs.
“At PartsSource, we’ve seen a 58 percent increase in the demand for depot repairs of the vital medical equipment used within ICUs, including infusion devices (large volume, syringe, PCA), feeding pumps, patient monitors and modules, telemetry transmitters, vital signs monitors, compression devices, EKG carts, defibrillators and pacemakers. As healthcare organizations ask clinicians to work at the top of their licenses, we believe supply chain leaders are asking the same of their clinical engineering departments — it’s not whether the engineers can fix items that need service, but whether or not they should fix certain devices. Hospital supply chains are starting to recognize additional cost savings, time-savings and improved quality implications by outsourcing depot repair.”
A new concept to healthcare organizations in and outside of the ICU is PartsSource Depot Repair — an ISO-certified offering for repair of 640+ equipment models of all major original equipment manufacturers (OEM). Typically, healthcare organizations have to manage many depot repairs through multiple service providers, which can take an average of 95 minutes per repair order. PartsSource provides these depot repair services all with flat-rate repair costs, up to a one-year no-hassle warranty and using a 60-second ordering process.
References:
- Critical Care Statistics, Society of Critical Care Medicine (SCCM), http://www.sccm.org/Communications/Pages/CriticalCareStats.aspx<
- Schrier, Robert W. and Wang, Wei, Acute Renal Failure and Sepsis, New England Journal of Medicine, July 8, 2004 http://www.nejm.org/doi/full/10.1056/NEJMra032401
- Sedation, Delirium, and Mobility in ICU Patients, Institute for Healthcare Improvement, http://www.ihi.org/Topics/SedationDeliriumMobility/Pages/default.aspx
- Catheter-associated Urinary Tract Infections (CAUTI), Centers for Disease Control and Prevention (CDC), https://www.cdc.gov/hai/ca_uti/uti.html
- 5. Peychl L., Zalud R., Changes in the urinary bladder caused by short-term permanent catheter insertion, Cas Lek Cesk. 2008;147(6):325-9.
- Beilan J, Lund T, Beane K, et al. Novel dual-balloon urinary catheters reduce catheter-associated urinary tract infections. Journal of Urology. 2016;195(4):e277. http://www.jurology.com/article/S0022-5347(16)01063-6/fulltext Last accessed: December 2, 2016.
- Borrego A, Kristov D, Elhaddah A, et al. Eliminating catheter associated urinary tract infections in the trauma ICU: two balloons are better than one. Presented at the Southeastern Surgical Congress Annual Scientific Meeting; Atlanta, GA; February 2016. Poster.
- Tampa General Clinical data overview for Duette Dual Balloon Indwelling Catheter System, Source: Poiesis.
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for prevention of catheter-associated urinary tract infections 2009. Centers for Disease Control (CDC), 2009.
- Helm, R. E., Klausner, J.D., Klemperer, J.D., Flint, L.M., and Huang, E. (2015). “Accepted but Unacceptable: Peripheral IV Catheter Failure.” Journal of Infusion Nursing, 38(3), 189-203
- Schell-Chaple, H. M., Liu, K. D., Matthay, M. A., & Puntillo, K. A. (2018). Rectal and Bladder Temperatures vs Forehead Core Temperatures Measured With SpotOn Monitoring System. American Journal of Critical Care,27(1), 43-50. doi:10.4037/ajcc2018865
About the Author
Kara Nadeau
Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.