Maintaining air, steam, water quality simply elemental
By air, steam or water, microbes and contaminants can spread throughout healthcare facilities, including those areas that play a key role in patient care and safety. This includes the Central Service/Sterile Processing & Distribution (CS/SPD) department, where instruments are reprocessed, and the operating room (OR) and other procedural areas where patient care is delivered.
Because it is often difficult – if not impossible - to monitor effectively air circulating in a room and the water and steam used for cleaning and sterilizing instruments with the naked eye, the quality of these elements can sometimes be overlooked. Poor air, steam or water quality in a healthcare environment can endanger staff members and patients, and damage infrastructure, equipment and instruments.
Manufacturers of systems for air, steam and water quality management, mitigation and treatment in healthcare environments offer their insights into the challenges faced in this area, the impact of poor quality, and best practices for measuring and monitoring these critical elements.
Quality in the air
Air quality is quickly becoming the next frontier in infection prevention, explains Jim Dacek, Senior Product Manager, Surgical Solutions, STERIS.
“In recent years, healthcare has addressed room surfaces, hand hygiene, gowning, etc., in the OR, but there hasn’t been much emphasis placed on air as a primary source of contamination. Hospital staff members need to be aware of potential risks associated with air quality that is out of spec (temperature, humidity, particle counts, differential pressure, etc.) per American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) Standard 170-2017, Ventilation of Health Care Facilities.”
In the OR and other procedural areas, James Marsden, Ph.D., Executive Director of Science and Technology, RGF Environmental, notes how air-borne or particle-borne pathogens including bacteria, virus and mold, can result in nosocomial infections and reinfection rates. He points to a recent case where a hospital’s failed efforts to contain Aspergillus mold led to a one-month-old baby’s heart infection following open heart surgery, and the family’s subsequent lawsuit.1
“Poor air quality presents considerable risk to patients and staff through nosocomial infections,” said Marsden. “Airborne viruses like SARS-CoV-2 and influenza can cause patient and staff issues, as can air and surface born bacteria like MRSA, strep, C-diff, as well as mold and yeast issues in HVAC and air-conditioned spaces.”
Karen Hoffmann, RN, MS, CIC, FAPIC, FSHEA, an infection preventionist consultant for the Vidashield UV24 from Nuvo Surgical, echoes the risks.
“Contaminated air in CS/SPD has been a factor in surgical site infection outbreaks primarily involving mold and fungi (e.g., Curvularia),” said Hoffmann.
“CS/SPD staff, who must work in close contact with disinfectants and sterilant products, can also suffer from breathing respiratory irritants for prolonged periods.”
Specific to the CS/SPD, Oyvind Birkenes, CEO of Airthings, points to how harmful contaminants, such as airborne pollutants, particulate matter, bacteria and more, can undermine sterilization efforts if they are not removed from the air in the department.
“There’s a reason we refer to air quality contaminants as ‘the invisible enemy’ – cracks in the building’s foundation, outdated or poorly functioning HVAC systems, and windows that are not properly sealed (or conversely, left open for too long) can all carry air pollutants into an environment and make achieving sterilization difficult,” Birkenes explained.
“The goal of a hospital CS/SPD is to provide the right items to the user at the right time and under the right conditions,” Hoffmann added. “Providing a sterile item in the right condition to the end user’s area requires not only good work practices, but also good environmental conditions. The challenge for CS/SPD is to ensure that environmental monitoring systems are used routinely (according to the established standards) for proper quality assurance to ensure good environmental conditions.”
As Birkenes indicates, the impacts of poor air quality can also have a major impact on health and well-being of CS/SPD staff members. He references the risks of “Sick Building Syndrome,” which the Environmental Protection Agency (EPA) defines as:
“A set of symptoms that affect some number of building occupants during the time they spend in the building and diminish or go away during periods when they leave the building. Cannot be traced to specific pollutants or sources within the building.”2
“Poor air quality, caused by a broad range of factors like CO2, airborne pollutants, particulate matter and more, can lead to a number of detrimental outcomes ranging from triggering asthma and allergies all the way to radon-induced lung cancer and the transmission of airborne viruses,” said Birkenes. “Mold or mildew spores can also irritate the respiratory system.”
Birkenes says another contributing factor to poor air quality in the hospital environment is the heavy use of chemical cleaners, coupled with “notoriously dry air” that increases the risk for chemical exposure and virus transmission due to lower-than-recommended humidity levels.
“Bottom line, if you don’t know what’s lurking in your air, you’re likely making it harder to achieve a sterilized environment,” Birkenes added.
Monitoring and measuring air quality
With an estimated 1 in 25 hospital patients suffering at least one healthcare-associated infection (HAI) each year, according to the Centers for Disease Control and Prevention (CDC),3 hospitals and other healthcare facilities have good reason to identify and address the factors increasing infection risk, including the quality of air in the OR.
“Air quality can have a big impact on preventing HAIs, so it is critical that patients are provided the best possible indoor air quality (IAQ), especially in the OR,” said Dan Diehl, CEO of Aircuity. “Key parameters to healthy air in the OR include Total Volatile Organic Compounds (TVOC), particles and dewpoint.”
Diehl explains that if dewpoint/humidity levels get too high, it can affect equipment performance by damaging circuit boards or other components which were not designed for those levels of humidity exposure. This in turn directly impacts the level of patient care a hospital provides.
To ensure CS/SPD departments have the right air quality ventilation measures and monitoring in place, NUVO Surgical’s Hoffmann suggests hospitals following indoor air quality standards, including ASHRAE guidelines 62.1 and 170 and ISO 14644. These specify that the clean air must be particle free, and the air-filter pore size should range between 0.05 µm and a maximum of ≤10 µm. She adds that tests for differential air pressure (minimum, 2.5 Pascals), air velocity (minimum, 2,500 cfm), air exchange rate (≥10 per hour) and air microbiology (measuring bacterial and fungal colony counts) are required to minimize air impurities in the sterile zone.
“The entire CS/SPD decontamination processes (from cleaning to sterilization) can only be safe if the surrounding environment is clean and controlled,” she said. “Air should always move from the sterile zone to the dirty zone to avoid cross contamination. Purified and moisture-free air is essential for preparing sterile materials and preserving them for a long time. An environmental quality monitoring system can reduce unnecessary sterilization cost and increase the shelf life of medical devices.”
Marsden recommends hospitals employ negative or positive pressure monitoring in isolation rooms, per CDC guidance; ATP (adenosine triphosphate) testing to show evidence of microbial activity for verification of cleanliness for critical equipment and areas; HVAC equipment monitoring operational and maintenance status; and general monitoring of indoor air quality (e.g., temp, humidity, particle counts and evidence of microbial activity).
Birkenes says healthcare facilities can leverage technology solutions for continuous monitoring of CO2, radon, PM 2.5 and 1, airborne chemicals, virus risk, mold risk, and many more factors that can lead to contamination.
“By monitoring for these factors constantly, CS/SPD departments can achieve an informed and data-rich knowledge base of what needs to be addressed specifically and where the issue may be stemming from,” Birkenes said. “Once that’s determined, they can take more aggressive action, such as using air sterilization techniques like Ultraviolet Germicidal Irradiation (UVGI) – but before that can happen, it all starts with diagnosing the problem.”
“Facility engineers can extract the data and present graphical reports during their monthly meeting with infection control,” said Diehl. “When a question about the condition of a particular space arises, data can also be brought to doctors, nurses and infection control teams to determine whether air quality had any influence. Staff can then focus on the clinical side, getting to the root of the problem.”
Using UV to disinfect the air
As the U.S. Food and Drug Administration (FDA) states, ultraviolet (UV) radiation has effectively been used for decades to reduce the spread of bacteria and may also be effective in inactivating the SARS-CoV-2 virus.4 Healthcare facilities have been implementing a variety of hospital-grade UV technologies to disinfect the air and surfaces.
When updating its handbook, ASHRAE convened Technical Committee, 2.9, Ultraviolet Air and Surface Treatment to clear up misconceptions regarding UV technologies, with the Committee members expressing the hope that the “pandemic has left no doubt that the 254 nm germicidal wavelength (UVC light) can inactivate the genetic material in the SARS-CoV-2 virus.”5
American Ultraviolet offers a wide range of UVC solutions, including mobile UVC, fixed mounted surface and air disinfection units, and upper room UVC for occupied spaces including all HVAC applications, all made in America in the company’s main factory in Lebanon, Indiana.
“Our Fixed Mounted UVC packages have been very popular in healthcare,” said Katja Auer, Clinical Director of Healthcare Solutions at American Ultraviolet. “These packages are unique in that they provide effective and fast cycle times, are easy to use, offer daily automated cycle times, and do not require additional FTEs. They are found in operating rooms, surgical suites and any areas that benefit from adjunct UVC cleaning in-between patients or procedures and/or after terminal cleaning in an operating suite took place.”
Since the COVID-19 pandemic began, American Ultraviolet has been working with multiple robotics companies to develop “truly autonomous UVC robots” for use in healthcare, where they have worked with clients to design and build mobile N95 mask disinfection solutions, and several government agencies to provide effective solutions for air and surface disinfection.
“What makes American Ultraviolet so unique is simply the people working for the company,” Auer added. “The last two years have really been a push trying to help and provide as many solutions for as many people/environments as possible and we asked a lot of our team, the admin or ‘front of the house’, engineering, production, sales, and all continuously work hard to deliver solutions. They put in the extra time knowing how important their work is, to help to keep people safe.”
R-Zero is a biosafety technology company that provides smart, sustainable disinfection to improve productivity, reduce sick days and enable better health through “clinically clean” shared spaces. As Natalie Quinn, Director of Brand and Content describes, R-Zero is devoted to enabling better outcomes for patients and providers:
“R-Zero takes a holistic approach to indoor environmental health by understanding the risk factors of a given hospital or health system’s spaces and then identifying how our technology can mitigate that risk while improving indoor air quality (IAQ) and eliminating pathogenic threats. We believe clinically clean shared spaces are achievable with less waste and less use of harmful chemicals, and we are deeply committed to enabling a new normal where elevated indoor health is the status quo.”
R-Zero’s Arc UV system enables hospital-grade efficacy while emitting 109% more light at a lower cost than competitors. With Arc’s powerful lighting array and overall design, the device can disinfect the air and surfaces in a 1,000 square foot room in just seven minutes. Arc also has LTE-M connectivity to R-Zero Connect, the company’s dashboard that allows users to track disinfection cycles, thereby making the traditionally unseen disinfection process visible and auditable.
“Simply put, we kill COVID - and a host of other common microbial threats,” Quinn commented. “R-Zero’s disinfection ecosystem enables safer indoor environments by destroying 99.99% of pathogens (including coronavirus, E. coli, the common cold, and the flu). As our products disinfect spaces in mere minutes, they eliminate the need to use harmful chemicals - all while creating a clear record of disinfection activity via our software dashboard. Implementing R-Zero’s suite of solutions will enable you to fight COVID today and HAIs or any other emergent threats tomorrow.”
Quality in water and steam
The Joint Commission has made water quality a priority, as evidenced by its New Water Management Requirements issued March 19, 2021. Under the new requirements, a hospital must have a water management program that addresses Legionella and other waterborne pathogens, and an individual or team responsible for the oversight and implementation of the program, including but not limited to, development, management and maintenance activities.6
Water quality is a key consideration for the CS/SPD department in the processing of instruments for re-use, from water used in decontamination sinks for pre-cleaning and rinsing, to the water used to generate steam for sterilization.
Steven Autiello, Regional Manager, Barclay Water Management, explains how the maintenance of water treatment equipment and monitoring of water quality presents several challenges to healthcare organizations and their CS/SPD departments.
“Rinse water used in sterile processing should be pretreated with a de-ionized (DI) or reverse osmosis (RO) treatment system to remove organics and inorganics from reaching instruments. Normally SPD departments handle the operation of this equipment, or it is done by a third party. Steam purity is typically at the mercy of the hospital’s central steam system, which would originate from a central steam plant in the hospital over which SPD has no control.”
Monitoring and measuring water and steam quality
“Checking bacteria and endotoxin levels are the best way to measure and monitor to understand the water quality,” said Khoukaz. “Other measurements to consider are pH, conductivity and water hardness. The first step for water purity concerns would be to assemble a team and do swab and water testing to establish current levels. If levels of water contaminants are higher than acceptable levels, various types of filtration can be used to address different problems.”
To overcome the challenges of water treatment and steam generation performed outside of the CS/SPD department, Autiello has seen hospitals install dedicated equipment for the pre-treatment of rinse water and steam generators specifically for sterilization within the sterile processing area.
“Properly maintained air, water and steam is nothing new to the industry so there are no silver bullets, though diligence in maintaining the installed pretreatment equipment is crucial,” said Autiello. “Try to identify the issue (air, water or steam) based on evidence or perceived issue. Work with facilities to correct or third-party vendor, if applicable.”
References
1. Mold found in baby’s heart after surgery; family suing Seattle Children’s hospital, Seattle Times, April 14, 2020, https://www.seattletimes.com/seattle-news/health/after-mold-infects-babys-heart-another-family-sues-seattle-childrens-hospital-over-ongoing-aspergillus-problems/
2. Sick Building Syndrome, EPA, https://search.epa.gov/epasearch/?querytext=sick+building+syndrome&areaname=&areacontacts=&areasearchurl=&typeofsearch=epa&result_template=#/
3. Healthcare-Associated Infections (HAIs), CDC, https://www.cdc.gov/winnablebattles/report/hais.html
4. UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus, FDA, February 1, 2021, https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/uv-lights-and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus
5. Behind the Update: ASHRAE Handbook Chapters on UV-C to Include Updated Best Practices, Guidance, ASHRAE, https://www.ashrae.org/news/ashraejournal/behind-the-update-ashrae-handbook-chapters-on-uv-c-to-include-updated-best-practices-guidance
6. The Joint Commission, New Water Management Requirements, March 19, 2021, https://www.jointcommission.org/-/media/tjc/documents/standards/prepublications/water_management_prepub_hap.pdf
7. Lee, L. (2017). Surface and air: What impact does UV-C at the room level have on airborne and surface bacteria? CJIC, Vol 32, Issue 2, p.108-111.
Solutions/tools for better air, water and steam quality
With greater recognition for the impacts of air, water and steam quality on patient/staff safety, infection prevention and effective instrument sterilization, manufacturers have developed solutions for proactive treatment, monitoring and contamination remediation in healthcare environments.
Aircuity: Aircuity’s centralized, multi-parameter demand control ventilation system continuously measures and adjusts ventilation rates using science-based IAQ parameters, so ventilation levels are based on current conditions. This can be used not only in OR suites, but also in medical research buildings, vivariums and other variable population areas, such as a lobby or waiting room. Users can monitor analytics on ventilation rates using the solution’s dashboard, helping facilitate compliance with external regulatory reporting.
In the OR, a solution called Clean Standby Mode continually measures dry bulb temperature, dewpoint/humidity and optimizes unoccupied ventilation for energy savings during times when the OR suite is not in use. When any IAQ “event” is detected and when the OR is in use, ventilation rates run at full occupied flow. This solution provides clean air while helping to address hospital sustainability goals and utility budgets.
Airthings: The Airthings for Business solution provides healthcare organizations the ability to continuously monitor a very wide range of air quality levels in as many rooms, buildings or campuses as they desire, including procedural areas and the CS/SPD. Armed with detailed air quality information, facility managers can optimize HVAC settings and ensure optimum air quality conditions. The Airthings Dashboard allows facility managers remote access to live air quality data across all monitored locations at once.
Through Airthings API, a healthcare facility can also integrate with building management systems to automatically trigger HVAC systems to turn on/off based on actual air quality data, ensuring that people are kept in a healthy environment while also reducing energy usage in buildings.
American Ultraviolet (AUV): AUV’s Fixed Mounted UVC packages deliver disinfection energy from multiple points on the ceiling, which limits or eliminates shadowed areas. The system is always available at the “push of a button”, offers fast cycle times and eliminates having to track down and move a mobile UVC robot. The daily auto-disinfection cycle can be scheduled to run when needed, such as one hour before treatment/surgical suites are opened, or on a prescheduled 30-, 45-, or 60-minute cycle. All disinfection cycles and operational data reports can easily be downloaded and shared with staff on a daily, weekly or monthly basis.
Barclay Water Management: As a water treatment company, rinse water and steam treatment is one area of Barclay Water Management’s expertise using pretreatment equipment and chemicals as its tools to provide solutions. Other services/solutions include:
- Chemical treatment programs for cooling towers, boilers, closed loops, specialty systems and wastewater
- Water management plans and consulting
- Water hygiene products and services for minimizing the risk associated with Legionella bacteria, the bacterium responsible for Legionnaires’ disease, in building potable water systems with an emphasis in healthcare
- Environmental cleaning services such as cleaning services for cooling tower, ice machines, hot and cold water storage tanks, decorative fountains and air handlers
- SAFE system storage and feed systems designed to eliminate operator handling of water treatment formulations, eliminating problems associated with handling and dispensing of treatment chemicals and the disposal of empty water treatment containers
- Service Tracking and Reporting Program (STAR) Internet-based application through which customers can access water test results and state-of-the-art software packages for data logging and remote communication with water treatment feed and control equipment
Nuvo Surgical: The Nuvo Surgical Vidashield UV24 adjunct air purification system uses ultraviolet germicidal irradiation (UVGI) to deactivate airborne bacteria and other organic pollutants, like mold and certain viruses. The Vidashield UV24 system can be incorporated seamlessly into existing overhead lighting or new construction and operates automatically by individual control decreasing the many issues with free standing floor units. The system pulls contaminated air directionally up to the ceiling as opposed to wall or floor HEPA /UV portable units that pull contaminated air across the room potentially exposing individuals in the pathway.
Improved ventilation using an adjunct UVGI system, like the Vidashield UV24, has demonstrated other benefits for healthcare workers, including improving the overall smell in the area and decreasing respiratory irritants and allergens that come from patient care procedures and enclosed environments.7
Pall: Pall Kleenpak capsule filters with intrinsically hydrophilic positive zeta potential Posidyne membrane are rugged, self-contained sanitary filters designed for small-batch sterile filtration of aqueous pharmaceutical solutions. With a net positive charge in most aqueous solutions from pH 3 to 10, Kleenpak Capsules with Posidyne Membrane Assemblies can be effective in removing contaminants, such as cell debris and endotoxin smaller than the filter rating.
RGF: RGF offers a variety of tools for air quality monitoring and management, including:
- Rapid Recovery Unit air purification and odor control system for non-occupied spaces
- Lucidium CUV system for HVAC coil sanitation and germicidal UV-C air purification (eliminates mold and yeast on coils, bacteria and viruses in the air)
- Lucidium Polaris upper room UV system, in accordance with CDC COVID-19 guidance
- Proprietary PHI-Cell in-duct whole building air purifiers for continuous, no-touch air treatment with low level, airborne gaseous hydrogen peroxide
- U.S. Food and Drug Administration (FDA) 510(k) approved medical devices Microcon MAP and ExC7 units for airborne pathogen control and creation of negative or positive isolation rooms (pair with Accustat room pressure monitors)
R-Zero: The R-Zero Arc UV system enables hospital-grade efficacy while emitting 109% more light at a lower cost than competitors. Due to Arc’s powerful lighting array and overall design, the device can disinfect the air and surfaces in a 1,000 square foot room in just 7 minutes. Arc also has LTE-M connectivity to R-Zero Connect, our dashboard that allows users to track disinfection cycles, thereby making the traditionally unseen disinfection process visible and auditable.
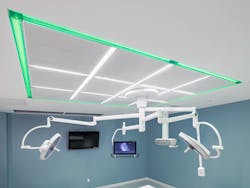
STERIS: The STERIS CLEANSUITE ceiling system helps mitigate the risk of airborne particles and prevents gaps within the room air unlike traditional laminar airflow systems. It is a true gapless laminar flow ceiling system, designed to minimize turbulence and gently guide particles away from the surgical site, bathing the patient and staff in ultra-clean air. At 100 particles per cubic foot, it delivers air quality aligned with the ISO Class 5 Cleanroom specification, surpassing the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) 170 standard for patient safety.
The flexible, modular design of the CLEANSUITE Ceiling System integrates structure, air delivery and room lighting. Internal and external truss systems allow for seamless mounting of surgical lights, booms, monitors, and c-arms. Prefabricated structural steel frame installs six times faster than traditional site-built ceilings to help maximize OR uptime. According to recent data provided by a national design and construction firm, CLEANSUITE is up to 40% less expensive than a traditional site-built ceiling.
General healthcare environments:
- American Society for Health Care Engineering (ASHE): https://www.ashe.org
- Centers for Disease Control and Prevention (CDC): https://www.cdc.gov
- Environmental Protection Agency (EPA): https://www.epa.gov
- The Joint Commission: https://www.jointcommission.org
CS/SPD department specific:
- Association for the Advancement of Medical Instrumentation (AAMI): https://www.aami.org
- International Association of Healthcare Central Service Materiel Management (IAHCSMM) https://www.iahcsmm.org
About the Author
Kara Nadeau
Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.