Inserting best practices with every catheter
Two years ago, Ben Galvan, an Infection Preventionist, worked in relative obscurity at Tampa General Hospital (TGH) in Florida. He audited quality care practices for central venous catheters (CVCs) and other medical devices, conducted surveillance for healthcare-associated infections (HAIs), developed procedures and trained staff to prevent infection transmission, and generally spread the good word of good hygiene throughout the hospital.
But after helping fight against HAIs through month after month of the pandemic, his job is now center stage, and Galvan’s team of eight (plus a director and manager) — are considered heroes.
In June, 2021, they received the 2021 Heroes of Infection Prevention Award from the Association for Professionals in Infection Control and Epidemiology (APIC). The award specifically honored the team’s Outstanding COVID-19 response.
Prior to COVID-19, “A lot of people might not have realized who we were,” Galvan said of the team’s role.
But during a time that has seen ICU beds filled, and unprecedented hospital staffing shortages, HAIs have been difficult to prevent. Patient-facing departments looked to Galvan’s work, and that of his colleagues, for information and recommendations.
Nationally, the Centers for Disease Control and Prevention (CDC) reported significant increases of HAI cases from 2019 to 2020. The usual suspects, including central line-associated bloodstream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), ventilator-associated events (VAE), each left its mark.
CLABSI in intensive care units (ICUs) saw a 50% increase in CLABSI cases in 2020 over 2019. During the especially hard-hit fourth quarter of 2020, cases increased by 65% over the fourth quarter of 2019. The same fourth quarter-to-fourth-quarter ICU comparison for CAUTI revealed a 30% increase. For VAE, ICU infection rates increased by 44%.
“We were in crisis mode for a long time,” Galvan said of COVID-19’s widespread impact. “Lots of patients had lots of (IV) lines and were incredibly sick.”
It was difficult for hospitals everywhere to prevent HAIs when they were packed with seriously ill patients requiring a high level of care, Galvan says. Staffing shortages left lower nurse-to-bed ratios than normal, and immunity-suppressing factors such as high steroid and antibiotic use raised the likelihood of infections.
The staff at TGH got a brief mid-fall respite when the volume of COVID-19 patients tapered off. But before the next big surge, a full-blown COVID-influenza “twindemic,” or another virulent mutant strain shows up, Galvan says it’s time, “to get back to the nuts and bolts,” of infection prevention.
He lists some of the most likely and dangerous places where bacteria lurk.
“IV pumps themselves can be a source of infection if not cleaned properly,” he said. “Central line care — the catheter itself — has to be secured, not tugging on the skin, which can cause micro-tears. We want to make sure our IV tubing is not expired and that it’s labeled properly. The dressing right around the (catheter) insertion site must be clean, and changed as needed.”
Preventing contamination of the insertion site is key, he says, and using anti-microbial dressings can help.
Pathogen-fighting products
Products designed to help prevent HAIs play an important role in infection prevention. Choosing them wisely can make a difference.
Teleflex makes antimicrobial vascular products that include the catheter itself. Chlorhexidine and silver sulfadiazine, “impregnate the entire indwelling surface length of the catheter,” according to the company’s description.
Arrowg+ard Blue Plus Protection, the line of CVC products offered by Teleflex, includes citations and summaries for research that has found the technology to be effective. Those include studies published in Association for Vascular Access (AVA), Intensive Care Medicine, and the American Journal of Infection Control.
Scott Schneider, Vice President of Sales for Teleflex, “are making the biggest impact on patient care and hospital performance.” The real benefits, he said, “are that they protect the patient while providing procedural efficiency for our clinicians.”
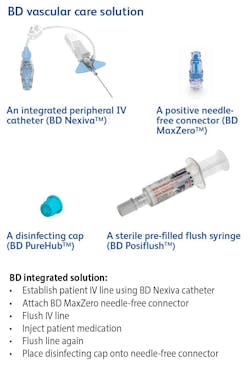
Becton, Dickinson and Company (BD) announced early last year (2021) results of a clinical trial, now published in The Lancet Infectious Diseases, which found that the risk of peripheral IV catheter (PIVC) failure can be reduced by 27% —resulting in longer catheter dwell times without complications — with four BD products, used in tandem.
“The problem with PIVC lines is that they often fail prematurely,” said Dr. Klaus Hoerauf, Vice President of Global Medical Affairs at BD.
PIVC re-insertions can be painful, and may raise the probability that additional complications will develop.
CDC guidelines recommend that PIVCs be replaced no more frequently than every 72 to 96 hours.
The CLEAN3 study, funded by BD but designed and conducted independently, included about 1,000 participants in a French university hospital. Both comparative dwell times and antiseptic solution effectiveness were examined.
“The whole goal of the study was to compare two peripheral vascular care approaches — an integrated product solution versus the hospital’s standard approach — in preventing complications that lead to peripheral IV catheter failure,” Hoerauf said.
CLEAN3 included the use of some of BD’s own products as part of that control group. They were used independent of a prescribed product combination.
The trial found the use of 2% chlorhexidine-gluconate (CHG) 70 % isopropyl alcohol (IPA) single use, sterile applicator skin, antiseptic reduced the risk of infectious complications by 92% compared with 5% povidone iodine (PVI) 69% ethanol.
The four-product regimen tested as part of the CLEAN3 trial includes PIVC (BD Nexiva), needle-free connector (BD MaxZero), disinfecting cap (BD PureHub) and a sterile prefilled flush syringe (BD Posiflush).
According to the trial summary, use of the integrated solution — or care bundle — is the best practice standard when peripheral IV catheter dwell time is expected to last more than 24 hours.
More than a billion PIVCs are inserted each year in hospitalized patients worldwide, according to the Journal of Hospital Medicine.3
Leadership is key
Not long after the TGH team was honored by APIC, Galvan was named as the organization’s inaugural “Emerging Leader in Infection Prevention.”
According to the commendation, Galvan, “managed performance improvement projects that have reduced the risk of patient harm and resulted in impressive outcomes such as a significant reduction in catheter-associated urinary tract infections, a reinvigorated hand hygiene compliance program, and a culture shift toward shared accountability with hospital cleanliness.”
Galvan shrugs off the accolade. After all, pathogen patrol is never done.
He’s sanguine about the future. People after all, may be wiser from having endured nearly two years of pandemic crises.
“Everyone is more savvy now in their understanding about healthcare,” he said.