In recent years, the focus of the Standard Practices column has often been on product identification and specifically unique device identification regulation. The efforts of manufacturers and more recently providers related to UDI compliance and adoption in the U.S. and now Europe has overshadowed the importance of the Global Location Number (GLN), which is the GS1 standard for organizations and locations. But the GLN is once again in the spotlight with publication of a new handbook on “Contract Communications Standards for the Healthcare Supply Chain” from the Health Industry Distributors Association (HIDA). The publication addresses one of the industry’s most vexing problems — ensuring price alignment among the many parties to a contract: manufacturers, distributors, group purchasing organizations (GPOs) and providers. Lack of alignment and resulting price discrepancies increase costs and complexity for all involved.
The causes of complexity are many, beginning with the myriad of contracts negotiated on behalf of providers by national and regional GPOs, as well as agreements reached directly between providers and manufacturers. According to the HIDA report, a manufacturer negotiates thousands of contracts with multiple business parties each year.
But contract negotiation is just the beginning. Contract execution creates even more challenges, in part because distributors often purchase products from manufacturers at a higher price than the contract price available to the provider to which they sell the product. Distributors recoup the difference by submitting chargebacks to manufacturers. A single manufacturer can easily receive hundreds of thousands of chargebacks from distributors every year. While chargebacks are a common practice, the business processes are anything but standardized. In fact, HIDA says the lack of standardized processes and data, along with minimal automation, are major contributors to cost and complexity.
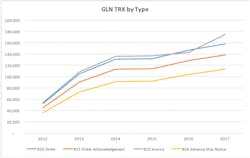
The HIDA report offers a full array of recommendations on how to automate and standardize processes, including which EDI transaction sets to use, how often, and what kind of data to include in each transaction. The paper notes that the U.S. FDA UDI rule has increased use of standardized product identifiers. On the other hand, there is no mandate that providers assign GLNs or other standard location identifiers to their ship-to locations. Also, unlike product identifiers, which must be assigned by the manufacturer, GLNs are the purview of providers. In other words, providers should be the ones to enumerate their various locations. In the absence of GLNS, providers must manage different numbers assigned by different suppliers for the same location. Assigning GLNS to those locations puts providers in charge and greatly minimizes the identifiers they must manage. Some GPOs have assigned GLNs for their provider members, but this can be less than ideal given that some providers have multiple GPOs and/or may switch GPOs over time. As the HIDA report points out, all parties to a contract will be able to use the same location identifiers in their communications, which can further reduce discrepancies among the parties. Unfortunately, after fairly strong adoption from 2012 to 2014, growth in the use of GLNs in basic transactions has leveled off. (See diagram)
Communications — both in terms of the method and timeliness — is another area where the HIDA report says we can make considerable improvement. The report recommends that all communications and transactions should be done electronically, ideally using electronic data interchange (EDI), but if not, then standard .CSV files matched to the EDI format. Specific EDI transactions include:
| Contract Process | EDI Transaction | From/To |
|---|---|---|
| Price Authorization Acknowledgements | 845 | Manufacturer to Distributor |
| Manufacturer Price/Sales Catalog | 832 | Manufacturer to Distributor |
| End User Sales Tracings | 867 | Distributor to Manufacturer |
| Chargeback/Rebate Reconciliations | 849 | Manufacturer to Distributor |
The HIDA report specifically states that distributors should send 867s with their chargeback/rebate claims to manufacturers within 45 days of the sale, and manufacturers should process those claims within 30 days of transmission. GS1 GLNs are strongly recommended to be used in 845s and 867s for both price authorization acknowledgements and end user sales tracings.
About the Author

Karen Conway
CEO, Value Works
Karen Conway, CEO, ValueWorks
Karen Conway applies her knowledge of supply chain operations and systems thinking to align data and processes to improve health outcomes and the performance of organizations upon which an effective healthcare system depends. After retiring in 2024 from GHX, where she served as Vice President of Healthcare Value, Conway established ValueWorks to advance the role of supply chain to achieve a value-based healthcare system that optimizes the cost and quality of care, while improving both equity and sustainability in care delivery. Conway is former national chair of AHRMM, the supply chain association for the American Hospital Association, and an honorary member of the Health Care Supplies Association in the UK.