July 2020 Product Picks
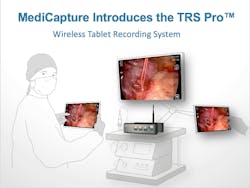
Wireless surgical imaging recording
MediCapture has launched the TRS Pro for wireless recording of surgical procedures. The first of its kind, the TRS Pro features MediCapture’s advanced MVR medical video recorder and a wireless tablet that can be connected to the recorder or used wirelessly throughout the operating room as a remote control or to stream video in real time. The tablet also can be used after surgery away from the operating room to review and manage patient sessions. TRS Pro’s non-computer based Smart Workflow interface powers MVR Manager, which enables surgeons to manage patient folders, edit videos and images, archive recordings and develop reports, and MVR Secure, which ensures safety of recordings. The system comes with full HD resolution and can be upgraded to 4K Ultra HD resolution or with DICOM.
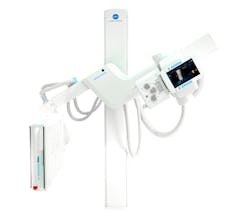
Digital orthopedic radiography
Konica Minolta Healthcare Americas, Inc. has introduced the commercial availability of the KDR Advanced U-ARM incorporated with the award-winning Dynamic Digital Radiography (DDR) for orthopedic imaging. KDR U-Arm with DDR recently received FDA clearance. DDR provides a series of individual digital images acquired at high speed and low dose. The resulting cine loop presents a diagnostic-quality view of anatomical structures while the patient is in motion, enabling orthopedic clinicians to observe changes over time. The digital cines can be enhanced, quantified, reprocessed, and replayed at normal, fast and slow motion, as well as one frame at a time. The KDR system performs standard X-rays and supports all common views, including weight-bearing studies.
COVID-19 mechanical ventilation
Enexor Health Systems, a wholly-owned subsidiary of Enexor BioEnergy, has been granted the U.S. Food and Drug Administration’s (FDA’s) Emergency Use Authorization (EAU) for the immediate delivery and use of the new X-VENT ventilator. The X-VENT is designed to deliver the critical modes of ventilation required for COVID-19 patients. It is one of the few FDA EUA-approved ventilators that does not use a bag valve mask resuscitator. It operates with a piston-driven air system controlled by a Schneider Electric industrial-grade, programmable logic computer. The system is self-calibrating, can easily be stored and is priced significantly below the cost of a traditional ventilator to encourage worldwide use. With the FDA approval, Enexor will ramp up manufacturing to meet the critical needs of hospitals in the U.S. and internationally.